Suspect Screening of Lupin-Produced Phytotoxins in Environmental Samples
Phytotoxins are secondary metabolites, typically < 1000 Da, synthesized by plants. The study of phytotoxins as endogenous plant hormones, dietary supplements, cosmetic ingredients, and pharmaceuticals dates back decades and is still of interest today.
Aristolochia clematitis or European birthwort. Branch with yellow flowers and green leaves | Image Credit: © kazakovmaksim - stock.adobe.com

Most recently, evidence of phytotoxins transferring from plant to terrestrial and aquatic environments has been reported, which demonstrates that these natural toxins are a new type of micropollutant in the environment (1–4). One example is Balkan endemic nephropathy, a disease that mostly occurs in the rural Balkan regions, and is linked to aristolochic acids, the nephrotoxic phytotoxins produced from Aristolochia clematitis. In this case, aristolochic acids were reported as persistent groundwater contaminants in Serbia, with groundwater being the major source of cooking and drinking water for the local residents (1).
Analyzing phytotoxins in environmental matrices such as soil, groundwater, and surface water is challenging. The compounds are structurally highly diverse. Their environmental occurrences and accumulations can be influenced by many factors such as vegetation, geographical conditions, agronomic factors, as well as season, weather, and climate change (5,6). In addition, the lack of reference standards and informative databases makes it difficult to perform qualitative analysis (screening and compound identification) and quantitative analysis (concentration measurement).
A robust, reliable, and efficient approach, namely Source Supported Suspect Screening (4S), has been introduced for high-throughput analysis of a wide range of phytotoxins, from their plant origin to the downstream environmental compartments, such as soil, groundwater, and surface water. The approach was demonstrated in a field study of Lupinus angustifolius (also known as blue lupin). This plant is a promising alternative to soya beans in European farming systems to provide food and feed; it is also a popular green manure in marginal lands (7). A limited number of lupinderived phototoxic compounds, mainly alkaloids and flavonoids, have been reported in agricultural soil and soil pore water (8,9). In this study, it was hypothesized that there could be a much higher number of phytotoxins transferred from the lupin plant to the surrounding soil and water environments. Based on this hypothesis, 106 plant, soil, drainage, and stream water samples were collected on a regular basis before, during, and after the growth cycle. These were analyzed through a 5-month agriculture cultivation of blue lupin in northern Switzerland.
This work has been reported previously in the journal of Environmental Science & Technology (10).
Experimental and Discussion
Sample Preparation for Plant, Soil, and Water
In the study, 41 lupin plant samples and 26 root soil samples were collected and extracted following a previously reported selective pressurized liquid extraction (sPLE) method (11). Extractions were performed on an ASE (accelerated solvent extraction) instrument (Dionex Corporation) under high pres - sure (1500 psi). The high pressure allowed the liquid extraction (50% aqueous methanol) to be performed at an elevated temperature (100 °C) with high recovery yields (67–103% for eight reference analytes, that is, convallatoxin, κ-strophanthidin, digoxin, digitoxigenin, odoroside A, proscillaridin, bufalin, and withanolide A) (11).
In comparison to other extraction methods, such as Soxhlet and ultrasonication extractions, sPLE has several advantages, including being programmable, automated, protective to light-sensitive and easily oxidized analytes, and in-cell cleanup. The sorbent layer (C18) on the bottom of the extraction cell not only adds selectivity but also enables the extraction-filtration cleanup to be achieved in one step. Key findings in the sPLE study (11) showed that the conventional heating step prior to solvent introduction caused significant thermal degradation (observed as reduced recovery) of a specific phytotoxin, digoxin, whereas a heating step after solvent introduction did not. Furthermore, matrix effects in the subsequent liquid chromatography–electrospray ionization-mass spectrometry (LC–ESI-MS) analysis were highly dependent on the sPLE dispersant used (Ottawa sand, diatomaceous earth, or glass beads), with the use of glass beads resulting in insignificant matrix effects. The matrix effects were also dependent on the combination of solvent strength and extraction temperature; the selection of 50% aqueous methanol and 100 °C kept matrix effects to a minimum, with an absolute average of 10% (σ = 4%).
In parallel, 30 drainage water and nine stream water samples were collected from the field itself (drainage water) and downstream from the field (stream water). These samples were concentrated following an established multilayer solid-phase extraction (mSPE) method reported by Mechelke and associates (12). The four-layer sorbents provided a broad coverage of analytes ranging from highly polar to nonpolar (logDow,pH7 -14–8), with overall good recovery (> 70%) and low matrix effects (63–72% on average), according to Mechelke and associates. and Günthardt and co-authors. (12,13).
Instrumental Analysis
The analysis was performed on an ultrahigh-performance liquid chromatography–electrospray ionization–quadrupole-time-offlight-mass spectrometryE (UHPLC– ESI-QTOF-MSE) analytical platform (Waters). A reverse-phase-based charged surface hybrid (CSH) column was chosen for its wide selectivity and good peak shape for basic compounds (14). Prior to batch analysis, chromatographic factors such as mobile phase pH/additive, organic modifier, column temperature, and gradient time were evaluated (15).
gradient time were evaluated (15). Results showed 5 mM ammonium formate (pH 6.5) as mobile phase additive and methanol as organic modifier significantly improved the overall detection sensitivity compared to the popular 0.1% formic acid and acetonitrile recipe. The optimal column temperature was set to 25 °C to avoid thermal degradation of phytotoxins during the chromatographic separation (Figure 1). The optimal gradient time was 30 min as a balance of peak capacity, peak production rate, and the total analysis time. ESI+ has been shown to efficiently ionize alkaloids, flavonoids, and many other phytotoxins and was therefore selected as the ionization mode (15). The LC–MS data were acquired using the data-independent-acquisition (DIA) mode where all precursor ions were fragmented without pre-selection. DIA is advantageous in terms of higher sample coverage and better reproducibility between samples, allowing the detection, structural characterization, and quantification to be achieved as an all-in-one analytical run (16).
Figure 1: Extracted ion chromatograms of digitoxigenin, bufalin, genistein, and artemisinin. Column temperature was set at 25, 35, and 45 °C. The mobile phase was (A) water and (B) 95% methanol, both with 5 mM ammonium formate as additive and prepared at pH 3.0. Flow rate was 0.3 mL/min. The UHPLC program was 0–1 min 1% B followed by linear gradient to 100% B in 10 min. The y-axes are linked for each of the phytotoxins.

Suspect Screening by the 4S Approach
For suspect screening, a list containing 733 phytotoxins was created. The list was a combination of 516 prioritized phytotoxins from the Toxic Plants−PhytoToxins database (17) and 217 phytotoxins from literature reports of secondary metabolites derived from Lupinus spp. (18–20). As demonstrated in Figure 2, raw LC–MS data were divided into two datasets: (i) a source plant dataset and (ii) an environmental fates (soil/water) dataset.
Figure 2: Flow-diagram of the Source Supported Suspect Screening (4S) approach. Phytotoxins detected in the source plant were used to improve the identification of phytotoxins in soil and water.
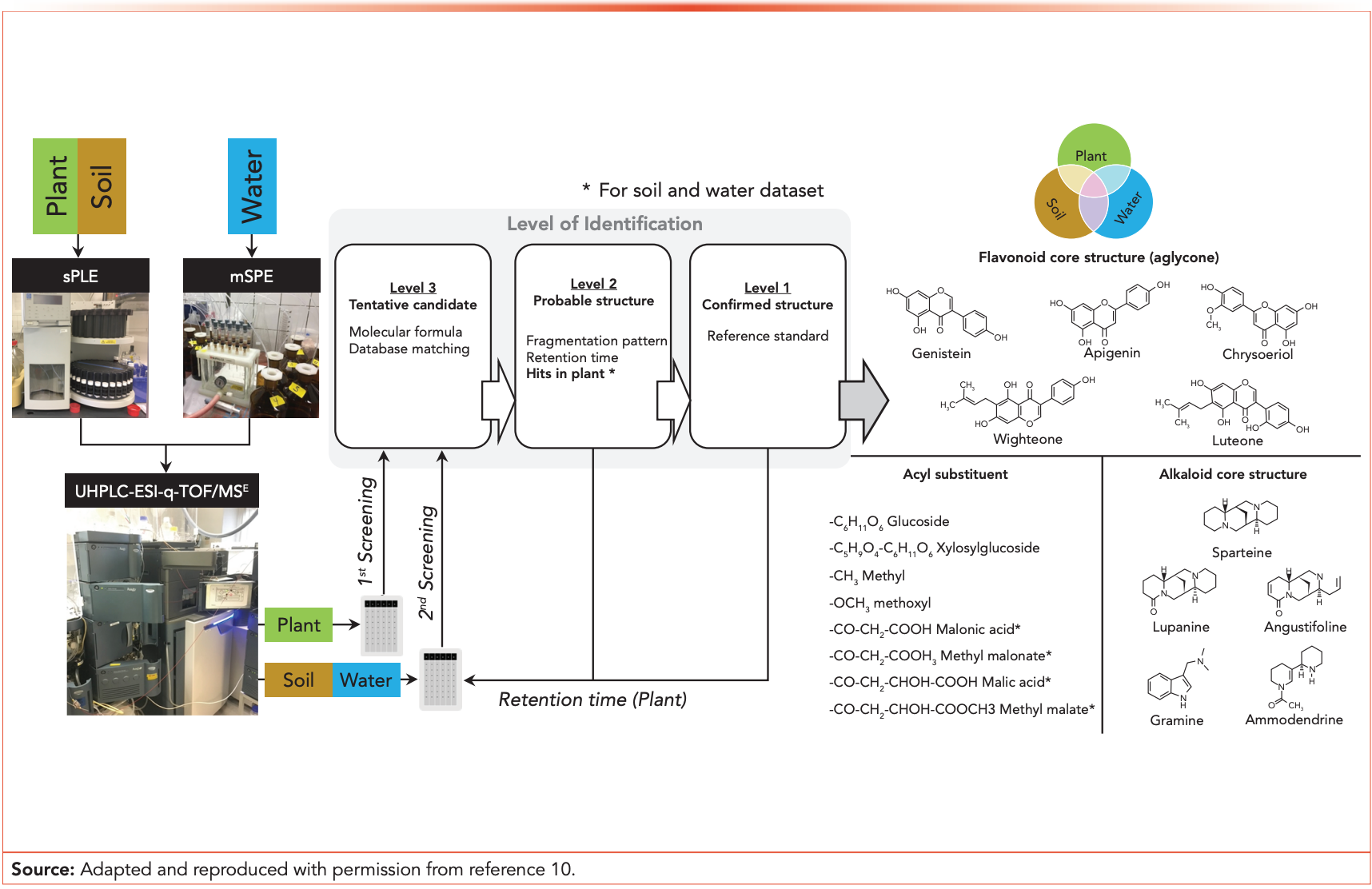
A suspect screening was first performed on the plant dataset, which yielded a list of 66 tentatively identified phytotoxins in the lupin plant. The initial suspect screening list was subsequently updated by including the LC retention time of phytotoxins identified with high confidence (level 2) and phytotoxins confirmed by reference standards (level 1).
The second suspect screening was performed on the soil/water dataset, where the updated suspect screening list was used. The retention times obtained from the plant dataset were included together with MS/MS fragmentation patterns as one of the level 2 identification criteria for phytotoxin candidates occurring in the soil/water matrix.
Phytotoxins in Plant, Soil, and Water
Using the 4S approach, 72 phytotoxins from the lupin plant and the environmental samples were identified with high confidence (level 1 and 2). As shown in Figure 2, they were exclusively flavonoids and alkaloids, mainly genistein, apigenin, chrysoeriol, wighteone, luteone, lupanine, sparteine, angustifoline, gramine, ammodendrine, formononetin, daidzein, caffeine, senecionine, retrorsine N-oxide, chelidonine, and their derivatives. The identities of genistin, genistein, gramine, sparteine, formononetin, daidzein, caffeine, senecionine, retrorsine N-oxide, and chelidonine were confirmed by comparison to reference standards. Sixty-six phytotoxins were consistently detected in the plant over its growing season.
Of the lupin-derived phytotoxins, 45 were detected in the soil, 34 were detected in drainage water, and 13 were detected in stream water. The results demonstrated that phytotoxins may be released from lupin and transferred to the surrounding terrestrial and aquatic environment. The established 4S approach can be powerful to assist in the discovery of phytotoxins in the environment.
Conclusion
As revealed in the study, the established 4S approach can be an efficient and reliable strategy to detect phytotoxins in multiple matrices, and can backtrack their occurrences from the downstream environments to the plant origin. In the long term, we expect the approach to assist in the discovery of more terrestrial- and aquatic-occurring phytotoxins, remedying the knowledge gap between their origin and environmental fate, and initiating related risk assessments and safety regulation.
References
(1) Tung, K.-K.; Chan, C.-K.; Zhao, Y.; et al. Occurrence and Environmental Stability of Aristolochic Acids in Groundwater Collected from Serbia: Links to Human Exposure and Balkan Endemic Nephropathy. Environ. Sci. Technol. 2019, 54 (3), 1554–1561. DOI: 10.1021/acs.est.9b05337
(2) Picardo, M.; Sanchís, J.; Núñez, O.; Farré, M. Suspect Screening of Natural Toxins in Surface and Drinking Water by High Performance Liquid Chromatography and High-Resolution Mass Spectrometry. Chemosphere 2020, 261, 127888. DOI: 10.1016/j.chemosphere.2020.127888
(3) Nanusha, M. Y.; Krauss, M.; Brack, W. Non-Target Screening for Detecting the Occurrence of Plant Metabolites in River Waters. Environ. Sci. Eur. 2020, 32 (1), 1–14. DOI: 10.1186/s12302-020-00415-5
(4) Hansen, H. C. B.; Hilscherova, K.; Bucheli, T. D. Natural Toxins: Environmental Contaminants Calling for Attention. Environ. Sci. Eur. 2021, 33 (1), 112. DOI: 10.1186/s12302-021-00543-6
(5) Bucheli, T. D. Phytotoxins: Environmental Micropollutants of Concern? Environ. Sci. Tech. 2014, 48 (22), 13027–13033. DOI: 10.1021/es504342w
(6) Günthardt, B. F.; Hollender, J.; Scheringer, M.; et al. Aquatic Occurrence of Phytotoxins in Small Streams Triggered by Biogeography, Vegetation Growth Stage, and Precipitation. Sci. Total Environ. 2021, 798, 149128. DOI: 10.1016/j.scitotenv.2021.149128
(7) Lopez-Bellido, L.; Fuente, M. Lupin Crop as an Alternative Source of Protein. Adv. Agron. 1986, 40, 239–295. DOI: 10.1016/S0065-2113(08)60284-9
(8) Hama, J. R.; Jorgensen, D. B. G.; Diamantopoulos, E.; et al. Indole and Quinolizidine Alkaloids from Blue Lupin Leach to Agricultural Drainage Water. Sci. Total Environ. 2022, 834, 155283. DOI: 10.1016/j.scitotenv.2022.155283
(9) Andersen, I. K.; Laursen, B. B.; Rasmussen, J.; Fomsgaard, I. S. Optimised Extraction and LC-MS/MS Analysis of Flavonoids Reveal Large Field Variation in Exudation into Lupinus Angustifolius L. Rhizosphere Soil. Rhizosphere 2022, 22 (4), 100516. DOI:10.1016/j.rhisph.2022.100516
(10) Liang, X.; Christensen, J. H.; Bucheli, T. D.; Nielsen, N. J. Source-Supported Suspect Screening (4S) of Phytotoxins in Terrestrial and Aquatic Environments: A Field Study of Lupinus Angustifolius L. (Blue Lupin). Environ. Sci. Techn. 2023, 57 (6), 2333–2340. DOI: 10.1021/acs.est.2c05387
(11) Liang, X.; Nielsen, N. J.; Christensen, J. H. Selective Pressurized Liquid Extraction of Plant Secondary Metabolites: Convallaria Majalis L. as a Case. Anal. Chim. Acta: X 2020, 4, 100040. DOI: 10.1016/j.acax.2020.100040
(12) Mechelke, J.; Longrée, P.; Singer, H.; Hollender, J. Vacuum-Assisted Evaporative Concentration Combined with LC-HRMS/MS for Ultra-Trace-Level Screening of Organic Micropollutants in Environmental Water Samples. Anal. Bioanal. Chem. 2019, 411, 2555–2567. DOI: 10.1007/s00216-019-01696-3
(13) Günthardt, B. F.; Schönsee, C. D.; Hollender, J.; et al. “Is There Anybody Else Out There?”-First Insights from a Suspect Screening for Phytotoxins in Surface Water. Chimia 2020, 74 (3), 129–135. DOI: 10.1016/j.scitotenv.2021.149128
(14) Fountain, K. J.; Hewitson, H. B.; Iraneta, P. C.; Morrison, D. Practical Applications of Charged Surface Hybrid (CSH) Technology. Waters Application Note 720003720EN, 2010.
(15) Liang, X.; Christensen, J. H.; Nielsen, N. J. Enhancing the Power of Liquid Chromatography–Mass Spectrometry for Chemical Fingerprinting of Phytotoxins in the Environment. J. Chromatogr. A 2021, 1642, 462027. DOI: 10.1016/j.chroma.2021.462027
(16) Wang, R.; Yin, Y.; Zhu, Z. -J. Advancing Untargeted Metabolomics Using Data-Independent Acquisition Mass Spectrometry Technology. Anal. Bioanal. Chem. 2019, 411, 4349–4357. DOI: 10.1007/s00216-019-01709-1
(17) Günthardt, B. F.; Hollender, J.; Hungerbühler, K.; Scheringer, M.; Bucheli, T. D. Comprehensive Toxic Plants–Phytotoxins Database and Its Application in Assessing Aquatic Micropollution Potential. J. Agr. Food Chem. 2018, 66 (29), 7577–7588. DOI: 10.1021/acs.jafc.8b01639
(18) Wink, M.; Meißner, C.; Witte, L. Patterns of Quinolizidine Alkaloids in 56 Species of the Genus Lupinus. Phytochemistry 1995, 38 (1), 139–153. DOI: 10.1016/0031-9422(95)91890-D
(19) Erdemoglu, N.; Ozkan, S.; Tosun, F. Alkaloid Profile and Antimicrobial Activity of Lupinus Angustifolius L. Alkaloid Extract. Phytochemistry Rev. 2007, 6 (1), 197–201. DOI: 10.1007/s11101-006-9055-8
(20) Von Baer, D.; Saelzer, R.; Vega, M.; et al. Isoflavones in Lupinus Albus and Lupinus Angustifolius: Quantitative Determination by Capillary Zone Electrophoresis, Evolution of Their Concentration During Plant Development and Effect on Anthracnose Causing Fungus Colletotrichum Lupini. J. Chil. Chem. Soc. 2006, 51 (4), 1025–1029. DOI: 10.4067/S0717-97072006000400006
About the Authors
Xiaomeng Liang was a PhD student in the Department of Plant and Environmental Sciences at the University of Copenhagen, Denmark. Jan H. Christensen is a professor in the Department of Plant and Environmental Sciences at the University of Copenhagen, and a member of the EAB for LCGC Europe. Thomas D. Bucheli leads the research group Environmental Analytics at Agroscope in Switzerland. Nikoline Juul Nielsen is an associate professor in the Department of Plant and Environmental Sciences at the University of Copenhagen. Direct correspondence to: njn@plen.ku.dk

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











