Label-Free Characterization of the Reversible Self‑Association of Insulin
The Column
The degree and strength of self-association are critical quality attributes for insulin and its analogues. In this article we present the label-free determination of these quantities using composition-gradient multi-angle light scattering (CG-MALS), enabling the formulation of engineered insulin optimized for stability and efficacy.
Photo Credit: Juan Gaertner/Shutterstock.com
Daniel Some and Sophia Kenrick, Wyatt Technology Corp., Santa Barbara, California, USA
The degree and strength of self-association are critical quality attributes for insulin and its analogues. In this article we present the label-free determination of these quantities using composition-gradient multi-angle light scattering (CG-MALS), enabling the formulation of engineered insulin optimized for stability and efficacy.
Therapeutic insulin analogues, engineered for specific states of self-association, are opening new frontiers in the treatment of diabetes. Modifications to wild-type insulin molecules impact the self-association of these molecules, and in turn alter their pharmacokinetics and pharmacodynamics. The result is fast-acting drugs best suited for prandial regimens and insulin pumps or extended-release versions for onceâdaily dosage (1). The ability to quantify the affinity and oligomeric state of insulin’s reversible self-association (RSA) is central to developing efficacious analogues.
Few experimental techniques provide a complete characterization of RSA, entailing the determination of oligomeric state as well as binding affinity. One technique particularly applicable to RSA measurements in all types of biopharmaceuticals is composition-gradient multi-angle light scattering (CG-MALS), a label-free technique that readily determines, from first principles, oligomeric state and equilibrium dissociation constants (Kd) of simple or complex RSA phenomena.
Light Scattering Measures Interactions
While analytical biochemists and biophysicists normally think of MALS as a means of molar mass determination via coupling to size-exclusion chromatography (SEC-MALS) (2), MALS can also be combined with a composition-gradient system (CG-MALS) to characterize biomolecular interactions (3,4). It measures the weight-average molar mass at each composition or concentration prepared by the composition gradient system, and analyzes the results in terms of association models. The output of this analysis is the goodness of fit to the model and the binding affinity for each complex presumed to have formed. In a well-planned experiment, the data set will typically be fit well by a small set of association models, and if the interaction is simple (for example, monomer-dimer or monomer-tetramer equilibrium) then only by a single model. This is a result of the highly constrained equations that govern the relationships between molar mass, concentration, Kd, and light scattering intensity in CG-MALS.
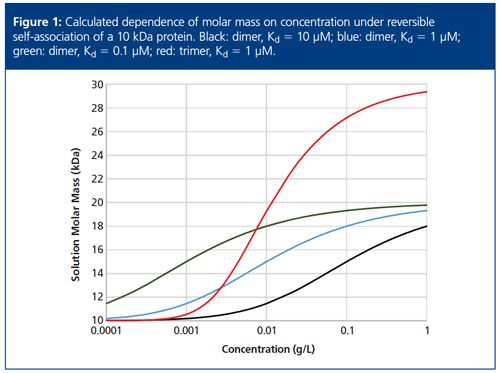
Conceptually, CG-MALS is straightforward and intuitive. If we assume a system is in dynamic equilibrium between monomers and a single type of oligomer, then at low concentration (that is, low relative to Kd), the molecules will be almost entirely dissociated, and MALS will measure the molar mass of the monomer. At high concentration (again, relative to Kd) the system will be mostly associated and MALS will measure the molar mass of the oligomer. Figure 1 illustrates the concentration dependence of the molar mass on the Kd value and the type of oligomer forming. If the system associates to form trimers, both the form of the dependence of molar mass on concentration and the absolute values of the solution molar mass at high concentration are quite different from those of the dimer.
Experimentally, a CG-MALS analysis consists of: quantifying the concentration dependence of the solution molar mass; identifying the association model that best fits the data; and producing the Kd value (binding affinity) of the interaction. The direct relationship between the molecular weights of the monomer and the associating complexes means that CG-MALS can determine not only the affinity but also the size of oligomers that form, even if more than one oligomer is in dynamic equilibrium with the monomers (for example, monomer-dimer-hexamer).
CG-MALS has been used to quantify the self-association of various macromolecules in solution, including insulin (3,4,5,6). While CG-MALS is also adept at analyzing hetero-associations (3,4), here we focus on its applications for homo-association, and specifically to analyze RSA of insulin.
Experimental
In this example, we quantified the selfâassociation of insulin under conditions of neutral pH and the absence of Zn2+. Human insulin samples were prepared in a buffer containing 20 mM sodium phosphate pH 7.2, 0.1 M NaCl, 1 mM EDTA. Experiments were performed at 25 °C in duplicate using two stock concentrations of either 3.2 mg/mL or 0.3 mg/mL.
CG-MALS experiments were performed using a Calypso II composition gradient system (Wyatt Technology) to create a concentration gradient, in line with a SPDâ6AV UV–vis spectrometer (Shimadzu) and a miniDAWN TREOS MALS detector (Wyatt). The light scattering and concentration data were fit to various self-association models using the Calypso software (Wyatt). Samples and raw data were kindly provided by V. Pandyarajan, N. Phillips, and M. Weiss, Case Western Reserve University.
Each concentration step was prepared by drawing aliquots of stock solution and diluent, and combining them on-the-fly via a static mixer at a flow-rate ratio that provided the desired concentration. The mixed samples were injected directly into the on-line detectors. Once the flow cells were filled, the flow stopped and the sample allowed to equilibrate. Data were acquired continuously, but typically only the equilibrium data were utilized in the final analysis.
Results
The CG-MALS method for RSA typically covers two or more orders of magnitude in concentration. The reduced data, after conversion of the light scattering and concentration signals to apparent molar mass, are characteristic of a selfâassociating molecule (Figure 3). At the lowest concentrations the molar mass is seen to approach that of monomeric insulin, ~6 kDa, while at the highest concentrations measured the solution weight-averaged molar mass is about 38 kDa - over six times higher than the monomer.
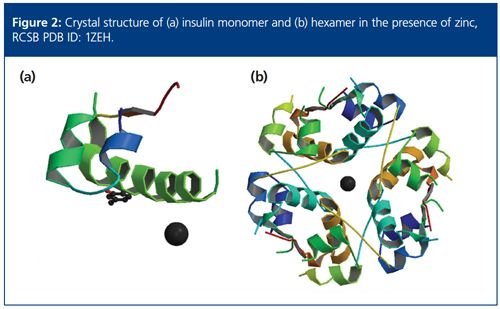
Naively, the ratio between the molar masses of the lowest and highest concentrations might lead one to conclude that insulin associates via a monomerâhexamer equilibrium; after all, that is a well known property of the molecule when in the presence of Zn2+. However, attempting to fit the data in the absence of Zn2+ with such a model will quickly disabuse us of this assumption. The dashed line in Figure 3 is the best fit of a monomer-hexamer model to the measurements, and clearly the fit is quite poor; it underestimates the measured Mw by as much as 30% for concentrations less than ~0.2 mg/mL (34 µM) and overestimates the Mw by as much as 25% for concentrations between 0.2 mg/mL and 2 mg/mL (34–340 µM).
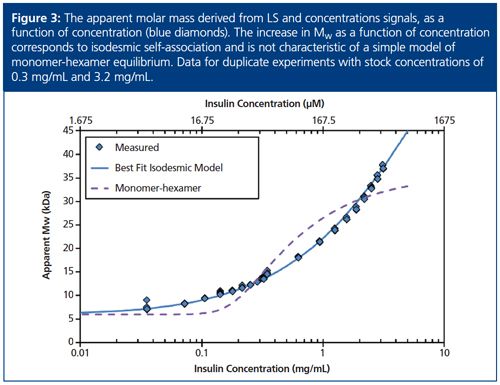
It is straightforward to test a series of association models against the data, and as it turns out, they are best fit by a model of “isodesmic“ self-association, indicated by the blue line in Figure 3. According to this mechanism, monomers self-associate to form dimers, trimers, and all higher-order oligomers. Each insulin monomer adds to a growing insulin cluster with constant affinity as follows:
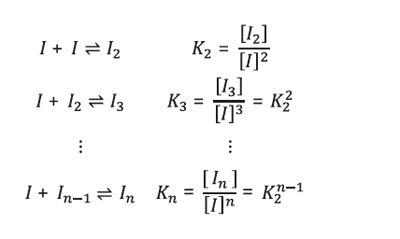
[1]
The equilibrium association constant K2 is related to the affinity per binding site by Kd = 1⁄K2. For the data in Figure 3, the best fit reveals isodesmic self-association with affinity KD = 52 µM and a monomer molar mass of 5.97 kDa.
These results compare favourably with literature data that utilized CG-MALS (3) and compared the results to sedimentation equilibrium, both of which found a Kd value of ~37 µM under similar buffer conditions. Kd does change with buffer pH, but for most conditions the association model remains constant. It is interesting to note that at the maximum concentration we tested (3.2 mg/mL, 536 µM), an adequate description of the insulin solution must include oligomers >10âmer. Higher concentrations would see correspondingly higher maximum oligomeric sizes.
Conclusions
Reversible self-association is a critical quality attribute of insulin and other biotherapeutic proteins, but not one typically given to direct quantification. Multi-angle light scattering, beyond its common use for analyzing molar mass and size distributions in the context of SEC-MALS, offers a powerful yet simple method to characterize RSA in terms of both affinity and the oligomeric state of complexes that are formed. Using CG-MALS we measured the isodesmic selfâassociation of human insulin in the absence of zinc, showing that insulin monomers form dimers, trimers, and higher order oligomers, with each insulin monomer adding to the growing cluster with equal affinity, and were able to quantify the affinity of this association. With the increasing availability of CG-MALS to developers of insulin analogues, it should be easier and faster to ascertain the desired formulation for optimal stability and pharmacodynamics.
References
- D.F. Berenson, A.R. Weiss, Z.I. Wan, and M.A. Weiss, Annals of the New York Academy of Sciences1243(1), E40–E54 (2012).
- P.J. Wyatt, Instrumentation Sci. & Tech.25(1), 1–18 (1997).
- D. Some, Biophysical Reviews5(2), 147–158 (2013).
- D. Some and S. Kenrick, Characterization of Protein-Protein Interactions via Static and Dynamic Light Scattering (Rijeka, InTech, 2012) p. 401–426.
- A.K. Attri, C. Fernández, and A.P. Minton, Biophysical Chemistry148, 23–27 (2010).
- A.K. Attri, C. Fernández, and A.P. Minton, Biophysical Chemistry148, 28–33 (2010).
Daniel Some is Principal Scientist and Director of Marketing at Wyatt Technology Corporation, where he has spent the last 12 years in various roles including R&D, software development, product management, and marketing. Previously Some was involved in the development of light-scattering–based patterned wafer inspection tools for the semiconductor industry.
Sophia Kenrick received her B.S.E. in chemical engineering from Arizona State University, Arizona, USA, and Ph.D. in chemical engineering from the University of California, Santa Barbara, USA. She is part of the applications team at Wyatt Technology, where she applies light scattering technology to solve problems in polymer characterization and biomolecular interactions.
E-mail:dsome@wyatt.com
Website: www.wyatt.com

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












