Key Factors in Sample Diluent Selection for HPLC Assays of Active Pharmaceutical Ingredients
LCGC North America
The authors explain why sample diluent is more than just a solubilizing agent and describe a systematic process for diluent selection when developing an HPLC assay of an active pharmaceeutical ingredient.
Sample diluent, a term familiar to analytical chemists, is defined as the vehicle that dissolves test material that will be assayed. The recipe for a sample diluent usually is determined empirically, and its complexity can range from a single solvent to a multicomponent mixture of solvents and solutes.

The goal of this article is to explain why the sample diluent is more than just a solubilizing agent and to recommend a systematic process for sample diluent selection.
The ideal sample diluent should have the following attributes:
- dissolves the major analyte
- dissolves impurities and degradation products
- is conducive to acceptable peak shape
- does not interfere with analyte response
- prevents analyte interaction with container surfaces, and
- does not promote analyte degradation.
Each attribute will be discussed in the context of high performance liquid chromatography (HPLC) assay development for active pharmaceutical ingredients, also referred to as drug substances.
Solubilization of the Major Analyte
Analyte solubility is the gateway and certainly the most obvious consideration for diluent selection. One has the advantage if solubility properties are known already, but for most drug candidates, such data might not be available. The ways to gain solubility information include practical understanding of organic chemistry, literature values for similar compounds, and empirical evaluation. Larger organizations probably have a pharmaceutics team that can provide such information to the method development group. Certainly the scientist must narrow down the choices for diluents based upon compatibility with the type of chromatography to be performed. If a compound is highly soluble in either acetonitrile and ethyl acetate, one would further explore the use of the former for reversed-phase separations but might find both solvents amenable to normal-phase chromatography.
Having a range of solubility above the target analysis concentration is recommended strongly, and threefold solubility is a bare minimum practical margin. To understand the importance of this margin, consider simple matters such as maintaining solubility when refrigerating sample solutions or when preparing higher concentration stock solutions. Refrigeration might be necessary for analyte stability, and sample precipitation would complicate the situation. Elevated sample concentrations often are applicable during method validations and when boosting column load for impurity identifications. As the project goes forward, the solid state or polymorph of the drug candidate can be changed such that it has altered solubility properties, and a good diluent choice may obviate changes to an analytical method. Although one cannot guarantee that any diluent will withstand every possible scenario, a devil's advocate mindset can pay dividends with regard to analyte solubilization. The recommendation is to establish more than one solvent system capable of solubilizing the analyte in order to have a back-up, because the diluent selection process has multiple criteria and flexibility might be necessary.
Solubilization of Impurities
It would not be sufficient to address only the solubility of the main analyte for diluent selection. One must anticipate the presence of impurities in the active pharmaceutical ingredient and be able to demonstrate that these are soluble in the selected diluent. Degradation products can be treated as impurities in this context. As drug development proceeds, new impurities in the drug candidate can suddenly render the diluent ineffective. To avoid such surprises, it would be prudent to use a sample diluent capable of solubilizing compounds having a range of polarity. For example, a major analyte might be highly water-soluble, and it would be tempting to employ water or buffer as the diluent for simplicity. Yet if an impurity having poor water solubility emerges during the project life cycle, it might be necessary to update the method with a new sample diluent. The choice of diluent is certainly case-by-case, but in this example of a water-soluble active pharmaceutical ingredient, the method developer can improve the odds against impurity solubility issues by incorporating 10–50% acetonitrile or methanol into the sample diluent.
An example of an unexpected impurity solubility problem is illustrated in a case study involving a zwitterionic water-soluble active pharmaceutical ingredient. The first batch of material, known to contain 0.1% of a nonpolar impurity, was dissolved easily at 1 mg/mL in a saline diluent. However, the subsequent active pharmaceutical ingredient batch earmarked for a clinical study would not form a clear solution in the saline diluent, and this raised major concern about the analytical method. Addition of a surfactant facilitated solubilization of the sample, and the level of the nonpolar impurity was found to be near 0.2%. That the haze resulted from this impurity was confirmed by before-after filtration comparisons. The lesson here is that the sample diluent was not robust, as evidenced by its failure above a threshold impurity level.
Impact on Chromatography
It is well documented that as the disparity in solvent strength between the sample diluent and the mobile phase increases, so does the risk of peak distortion and splitting (1,2). In simple terms, the distortion results from the moving sample zone of relatively high solvent strength that acts as an irregularity in the mobile-phase pump program. From this perspective, the ideal sample diluent is whatever mobile-phase composition runs through the column at the time of injection. Figure 1 illustrates an evaluation of three sample diluents for an amine analyte in a reversed-phase system and clearly shows the benefit of using low organic strength on peak shape quality. In this example, the main analyte is dissolved at the same concentration in three diluents having different levels of organic solvent. If one is forced to use high solvent strength in the sample diluent, for instance, to solubilize impurities, consideration should be given to decreased injection volume with possibly increased sample concentration so as to decrease the zone of differential solvent strength and to avoid compromising sensitivity.
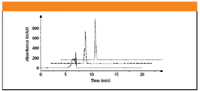
Figure 1: Effect of sample diluent organic solvent content on peak shape in a reversed-phase HPLC system. From top to bottom, the chromatograms reflect diluent containing 10% acetonitrile, 50% acetonitrile, and 100% acetonitrile. This figure shows offset retention times for illustration purposes.
The sample diluent should not exhibit interference with peaks of interest. It is also important not to ignore what happens in the void region. Solvents like dimethyl sulfoxide and acetone can be used as sample diluent components for reversed-phase methods, and because these exhibit a strong ultraviolet absorbance and tend to be eluted near the void, there is a risk of obscuring relevant impurity peaks from the test sample.
Analyte Stability
Analyte stability refers to the stability of an assay solution and must be evaluated as part of method development or validation (3). Degradation of the analyte by sample diluent should be avoided or significantly minimized in the context of the practical time window for sample preparation and analysis. One can predict situations in which lability will occur based upon fundamental knowledge of organic chemistry. It is appropriate to compare several diluents at ambient and refrigerated storage in relevant containers such as volumetric flasks and autosampler vials.
The experimental design can range from a simple run with repetitive injections to a multipoint weight percent quantitation. One should look for diminishing parent response and formation of new impurities over time. Given the typical variability in the parent response, it might be easier and more practical to track impurity formation. The ideal target is no more than 0.01–0.05% new impurities forming within the practical time window. Difficult compounds might require special measures such as frozen storage, the use of a chilled autosampler, and injection immediately following preparation. Figure 2 shows a simulated comparison of two sample diluents for their effect on analyte stability. In this example, Diluent A imparts reasonable analyte stability for at least 24 h, whereas the use of Diluent B would warrant sample preparation immediately before injection.
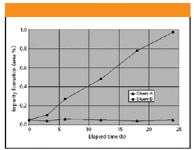
Figure 2: Simulated representation of the sample diluent effect on analyte stability.
Analyte–Surface Compatibility
The method developer can get bewildered when analyte recoveries appear too low or nonexistent upon sample dilution. Certain analytes in solution will interact with container surfaces such as volumetric flasks, pipettes, and autosampler vials. Positively charged and amphiphilic compounds typically exhibit such behavior with glass surfaces. If not addressed with the appropriate sample diluent, this phenomenon can influence analyte recovery. Given that there are a finite number of surface interaction sites in a container, the risk of compromising recovery increases as the analyte concentration decreases and as the surface area increases. Usually, one does not encounter recovery problems until the analyte concentration dips below 1 μg/mL. However, such low concentrations are common in cleaning verification assays and when using a diluted standard for sensitivity checks and impurity quantitation in drug substance release assays. It might be necessary to incorporate ionic strength into the diluent in order to compete against analyte for interaction with container surfaces.
One can evaluate the effectiveness of a sample diluent in preventing analyte–container interactions by performing a simple transfer experiment. A solution of the analyte near the target quantitation limit is prepared in a volumetric flask using the candidate diluent. An aliquot of a selected volume is immediately withdrawn and saved in an autosampler vial as the zero-transfer control. The remaining solution is then transferred to a second flask, and an aliquot is transferred from this flask into a second autosampler vial. The process is repeated several times to generate a set of vials representing the transfers plus the control. The samples are then analyzed by HPLC and a plot of peak area versus transfer number is drawn. A zero-order relationship indicates that no significant analyte–container interactions occurred and that the sample diluent is adequate for the method. A downward trend in peak area versus transfer indicates loss of analyte due to interactions with container surfaces. With a downward trend, it is a judgment call depending upon the slope magnitude. A very shallow slope might warrant no action, whereas a steep slope might indicate the need for evaluation of an alternate sample diluent.
Figure 3 illustrates a comparison between water-based and buffer-based sample diluents for an amine active pharmaceutical ingredient. Solutions of a proprietary amine were prepared at the 0.05% level in diluent containing water versus acidified phosphate buffer. A portion of each preparation was then transferred successively in parallel to five empty vials while withdrawing an aliquot from each transfer for analysis. The peak area response decreased approximately 60% after five transfers with the water-based diluent but remained steady with the buffer-based sample diluent. Separate controls confirmed that the observed loss with the water diluent system was not due to stability or solubility issues. The trends for each diluent support the hypothesis that ionic strength competes with analyte–glassware interactions. Separate controls confirmed that the observed loss with the water diluent system was not due to stability or solubility issues. The peak area response decreased approximately 60% after five transfers with the water-based diluent but remained steady with the buffer-based sample diluent.
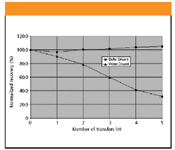
Figure 3: Effect of sample diluent composition on analyte recovery to evaluate compatibility.
Sample Diluent Selection Process
The selection of sample diluent should be suitable for intended use of the method. The scientist might have to accept the reality that no perfect diluent exists for some analytes. Convenience, practicality, and available research time should be balanced with what is necessary to give robust conditions.
A systematic approach to sample diluent selection is highly recommended, and here is an approach that addresses the key attributes:
- Select a diluent that solubilizes the active pharmaceutical ingredient at a minimum threefold excess of the target assay concentration and which is compatible with the chromatography mode. At the onset of chromatographic method development, the selection can be iterative with finding the assay concentration and column load that provide an acceptable detector response.
- Unless the selected diluent is already at full solvent strength, such as neat acetonitrile for reversed-phase HPLC, modify the initial diluent by increasing the solvent strength up to fivefold. Confirm that the modified diluent solubilizes the active pharmaceutical ingredient at a minimum threefold excess of the target assay, concentration as with the initial selected diluent.
- Establish a back-up sample diluent based upon solubility as described earlier. Set this information aside as a contingency should the first candidate prove unacceptable in subsequent evaluations.
- Store portions of the active pharmaceutical ingredient samples prepared in the initial and modified sample diluents refrigerated overnight. Confirm whether the samples stay in solution, and if not, then determine whether the precipitates can be solubilized at ambient temperature.
- Analyze the active pharmaceutical ingredient samples prepared in the initial and modified diluents and compare chromatograms. Confirm that the selected diluent does not interfere with quantitation of the main analyte or relevant impurities. If peak shape quality is compromised by the higher strength diluent, then further modify the diluent by backing off on the solvent strength. One should target the highest solvent strength that does not compromise peak shape quality as a hedge for new impurities or increased levels that appear in future lots. Confirm that the main analyte is not degraded significantly in the presence of the selected diluent, or at least establish a rough window of analyte stability. This is accomplished easily by letting the system perform repeat injections of the sample over a 6–24 h period.
- Evaluate the selected diluent for its ability to prevent analyte interaction with glassware and other relevant container surface types. Perform a transfer experiment with the active pharmaceutical ingredient at low concentrations. A practical target is 0.05–0.1% of the assay concentration, and three transfers are sufficient.
Following the process can pose challenging decisions. As an example, the best candidate diluent with regard to solubility, chromatography, and compatibility can promote poor analyte stability, and so the question can be raised whether it is worth having to prepare samples immediately before injection. Such measures might not be show-stoppers early in drug development, but it might be necessary to persist in the selection process for a better diluent as the project moves forward.
Conclusions
The examples provided in this paper illustrate that a sample diluent is more than just a solubilizing agent for the active pharmaceutical ingredient, and that one must consider the importance of the diluent for solubilizing impurities, acceptable peak shape quality, analyte stability, and analyte–surface compatibility. A systematic process for sample diluent selection is recommended.
Aside from the benefit to the active pharmaceutical ingredient analytical method, following a diluent selection process gives the scientist a wider knowledge scope that could benefit a drug development project. The establishment of back-up sample diluents can prove useful in the development of an orthogonal drug substance assay and drug product assays. Learning that the active pharmaceutical ingredient cannot be resolubilized once it has precipitated under refrigeration might hint at solid state polymorphism issues. Understanding the tendency for analyte to interact with container surfaces can come in handy for addressing how to extract analyte from cleaning verification swab samples. The observation of poor analyte stability in numerous diluent systems can influence decisions regarding toxicology study vehicles and parenteral dosage forms. The availability of such information to the project team would no doubt prove the diluent selection process valuable.
Acknowledgments
The authors would like to thank Dr. K.A. Babiak, Regis Technologies, Inc., for his technical input.
References
(1) C. Hawkins and J.W. Dolan, LCGC 21(12), 1134–1138 (2003).
(2) J.W. Dolan, LCGC 22(6), 524–528 (2004).
(3) ICH Q2B: Validation of Analytical Procedures: Methodology (International Conference on Harmonization of Technical Requirements for the Registration of Drugs for Human Use, Geneva, Switzerland, May 1997).
P.W. Wrezel, I. Chion, and R. Pakula are with Regis Technologies, Inc., Morton Grove, Illinois. P.W. Wrezel can be reached at pwrezel@registech.com
Reversed-Phases for LC Deliberately Doped with Positive Charge: Tips and Tricks for Effective Use
May 13th 2025In this month's edition of LC Troubleshooting, Dwight Stoll and his fellow researchers discuss both the benefits (improved peak shape/loading) and challenges (excessive interaction) associated with charge-doped reversed-phase (RP) columns for both analytical and preparative separations.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













