Investigation of Reequilibration in Hydrophilic Interaction Liquid Chromatography
LCGC Asia Pacific
Interest in chromatography using hydrophilic interaction liquid chromatography (HILIC) has continued to build in recent years. Adoption of the technique has been slowed by experiences of poor reproducibility. In particular, reequilibration times in HILIC have been reported as being exceptionally long as compared to reversed-phase chromatography. In this study, reequilibration times in HILIC for both aqueous–organic gradients and buffer gradients are systematically explored. The results not only promise to improve method development practices, but also provide insight into HILIC retention mechanisms across mechanistically differing polar stationary phases.
Daniel L. Shollenberger1 and David S. Bell2, 1MilliporeSigma, MerckKGaA, Darmstadt, Germany, 2Column Watch Editor
Interest in chromatography using hydrophilic interaction liquid chromatography (HILIC) has continued to build in recent years. Adoption of the technique has been slowed by experiences of poor reproducibility. In particular, reequilibration times in HILIC have been reported as being exceptionally long as compared to reversed-phase chromatography. In this study, reequilibration times in HILIC for both aqueous–organic gradients and buffer gradients are systematically explored. The results not only promise to improve method development practices, but also provide insight into HILIC retention mechanisms across mechanistically differing polar stationary phases.
The interest in using hydrophilic interaction liquid chromatography (HILIC) has continued to build in recent years. The complementary nature to reversed-phase analysis, the chromatographic benefits conferred when analyzing polar and basic compounds, and the inherent compatibility with mass spectrometry (MS) electrospray ionization (ESI) are all driving forces towards greater adoption of the technique. Full acceptance, however, has been slowed by experiences of poor reproducibility. These issues arise both from the limitations of parameters chosen during method development and lack of system controls when designing a chromatographic experiment. Notably, reequilibration times in HILIC mode have been reported as being exceptionally long compared to those in reversedâphase mode. Although there are likely underlying fundamental differences in equilibration mechanisms that contribute to this phenomenon, the application of common reversedâphase operational practices to column equilibration could be a contributing factor for reproducibility issues.
This column instalment describes a study of system controls for reequilibration times in HILIC with a systematic exploration of both aqueous–organic and buffer gradients. Reproducibility of retention and selectivity were observed as a function of equilibration times following runs in each type of gradient system. Two typical HILIC stationary phases, bare silica and pentahydroxyl bonded silica, were evaluated with different sets of molecular probes designed to suggest possible mechanisms driving the equilibration process as a function of time. It is hypothesized that both short equilibration times between analyses and run conditions that extend outside the normal HILIC range, representative of poor control of the chromatographic system, will show the greatest issues with repeatability. The results not only promise to improve method development practices, but also provide valuable insight into HILIC retention mechanisms across mechanistically differing polar stationary phases.
Experimental
Organic–Aqueous Gradient Study: Gradients were run on bare silica (Ascentis Express HILIC, 10 cm × 3.0 mm, 2.7-µm, MilliporeSigma) and pentahydroxy stationary phases (Ascentis Express OH5, 10 cm × 3.0 mm, 2.7-µm). A set of polar neutral molecules (adenosine and cytosine) and a set of polar basic molecules (metanephrine and normetanephrine) were studied for each column and gradient condition. Two gradients were evaluated: 5–50% aqueous (gradient 1) and 5–25% aqueous (gradient 2). Reequilibration times were set at 2, 5, 10, 15, and 20 min. Mobile-phase A was 5 mM ammonium acetate in 95% acetonitrile, mobile-phase B was 5 mM ammonium acetate in 50% acetonitrile, and mobile-phase C was 5 mM ammonium acetate in 75% acetonitrile. In each case, the pH of the mobile phase was unadjusted. Gradient 1 ran from A to B in 10 min. Gradient 2 ran from A to C in 10 min. In all experiments, column temperature was held at 35 °C, flow rate at 0.5 mL/min, injection volume at 2 µL, and detection by ultraviolet (UV) absorbance at 254 nm. All measurements were made in triplicate.
Buffer Gradient Study: The buffer gradient study was run on the same columns listed above using the same parameters for temperature, flow rate, injection volume, and UV detection. In addition to the polar neutral mix and the polar basic mix, a nonpolar basic mix using the tricyclic antidepressants amitriptyline and nortriptyline was included. A gradient of 2–10 mM ammonium acetate was run with constant organic–aqueous composition. Mobile-phase A consisted of 2 mM ammonium acetate in 90% acetonitrile and mobile-phase B consisted of 10 mM ammonium acetate in 90% acetonitrile. Both mobile phases were pH adjusted to 7.0 (±0.05) with acetic acid (pH measured in the presence of organic and calibrated using aqueous standards). The gradient was run from 100% A to 100% B in 10 min. All measurements were made in triplicate.
Results and Discussion
Figure 1 shows the structures of the test probes used throughout the study. Adenosine and cytosine represent neutral polar molecules that should only interact with the stationary phase via partition or polar (dipole type) interactions. The metanephrines are both polar and positively charged under the conditions of the study and thus can interact via partitioning, polar, and ionic mechanisms. The tricyclic antidepressants (TCAs), amitriptyline and nortriptyline, are likewise positively charged, but relatively nonpolar. The TCAs, then, are expected to retain via ionâexchange mechanisms and to some extent polar interactions, but not via partition mechanisms. Acenaphthene is included as a void volume marker.
Aqueous–Organic Gradients
Plots of retention time versus organic–aqueous reequilibration time for the neutral probes on the bare silica phase are shown in Figure 2. For the steeper gradient (to 50% aqueous, gradient 1, Figure 2[a]) there exists some slight irreproducibility when the reequilibration time was set to 2 min. At 5 min and above, highly reproducible results were obtained as noted by the barely discernable three overlaid symbols. For the shallower gradient (gradient 2, Figure 2[b]), 2 min appears to be sufficient to generate reproducible results. It was initially expected that short reequilibration times would result in greater variation in retention, but that was not the case. It was also expected that running the gradient to a higher level of the aqueous component would require additional time for reproducible retention times to be measured. This effect was only slightly apparent at the 2 min time point. In both plots, the retention drifts upwards as reequilibration times increase; however, at each given time point, reproducible results are obtained. Increasing retention of neutral probes as a function of longer equilibration times may indicate that the preferential adsorption of water on the silica surface is slow. As more of the surface becomes solvated with the aqueous layer, polar neutral compounds such as adenosine and cytosine would be expected to partition more and thus show greater retention. For the pentahydroxy phase, both gradient 1 and gradient 2 yielded highly reproducible results at each time point (data not shown). Retention variation as a function of reequilibration times were the same in terms of direction as compared to bare silica, but significantly attenuated.
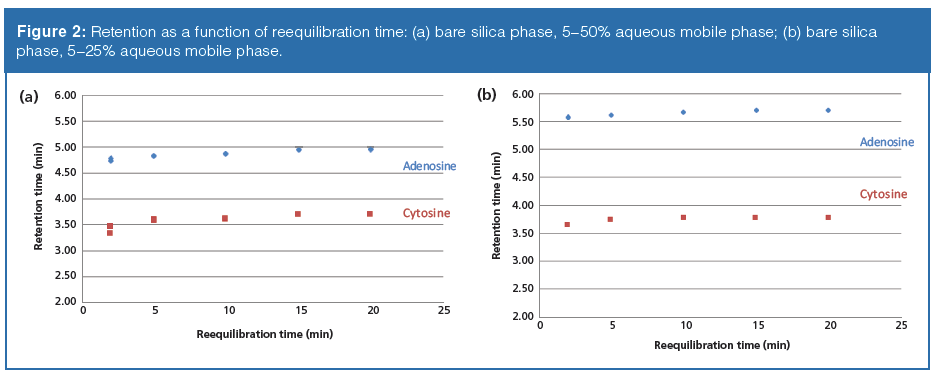
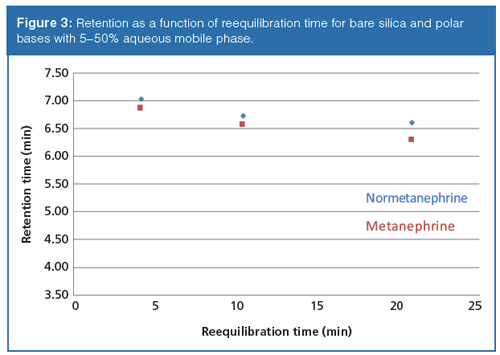
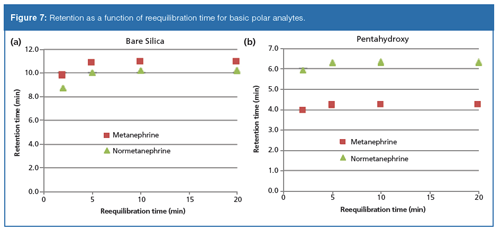
Figure 3 shows the results from the polar basic probes using gradient 1 on the bare silica phase. Similar results were obtained on the bare silica phase for gradient 2. Again, the retention times are shown to be highly reproducible at each reequilibration time studied. In contrast to the neutral probes, the polar basic compounds show a downward drift in retention as a function of reequilibration time. Since the polar basic probes may retain by both ion-exchange and partitioning mechanisms, interpretation of this retention trend is difficult. If ionâexchange is assumed to be the dominant mechanism of retention, it is conceivable that the slow preferential adsorption of water could potentially mask ionic interactions with the ionized surface silanol groups resulting in the observed reduction in retention with time.
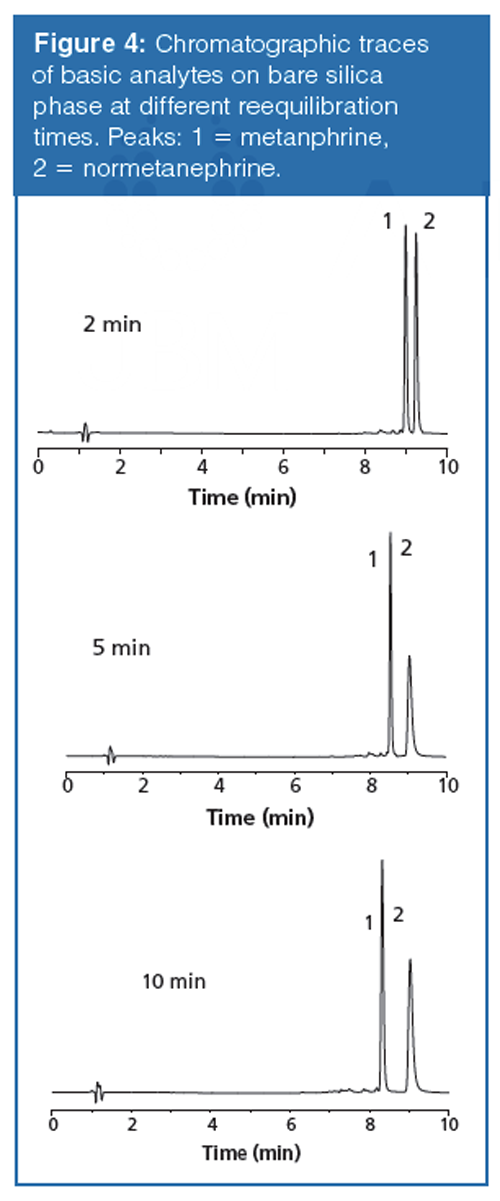
For the neutral polar analytes, relative retention and peak shapes on both stationary phases remained highly consistent throughout the study and reproducible as a function of reequilibration time. Figure 4, however, shows significant changes in selectivity and peak shape of the polar bases when run on the bare silica phase as a function of reequilibration times. These variations in retention and peak shape were still highly reproducible at each discrete reequilibration time. These chromatographic changes imply the analytes experience a different interaction with the stationary phase as a function of reequilibration. The observation suggests consequences for standard method development practices common in reversed-phase chromatography when applied to HILIC systems. In reversed-phase it is often assumed that after the system is equilibrated “enough”, that any time between injections beyond that time will result in reproducible data. The observations in this work demonstrate that care must be taken to ensure reequilibration times are considered as a significant parameter to be controlled during HILIC method development and expressly stated in the final method conditions.
In comparison, Figure 5 shows similar traces obtained for the polar bases on the pentahydroxyl phase, which indicates that the reequilibration time does not appear to significantly impact the retention, selectivity, or peak shape. In a previous study, bare silica phases were shown to exhibit moderate ionâexchange character and partitioning character whereas the pentahydroxy phase exhibited little ion-exchange and strong partition mechanisms (1). Comparison of the behaviour of the polar bases on both stationary phases points to the presence of both ion-exchange and partitioning mechanisms available on the bare silica phase that affect the chromatographic profiles. Further effort is required to determine the exact dynamics driving this observation; however, it appears that the more polar pentahydroxy phase may facilitate preferential solvation of the water on the surface. The expedited coverage and likely extent of the water associated with the surface reduces change with time and is expected to mediate any ionâexchange interaction potential.
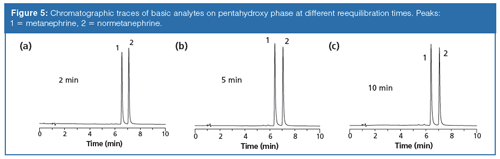
Buffer Gradients
To investigate the role of ionâexchange mechanisms in the reequilibration processes, buffer gradients were run on both phases and an additional set of nonpolar basic probes was added. The buffer gradients were held at constant aqueous–organic ratios to minimize the impact of changing the preferential solvation of the surfaces with the aqueous component. The addition of the hydrophobic bases to the set of probes was intended to better interrogate the ion-exchange mechanisms, as partitioning of these probes should be minimal.
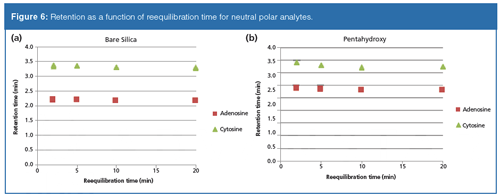
Figures 6–8 show plots of measured retention times versus buffer gradient reequilibration times on the bare silica and pentahydroxy phases. On both phases, the neutral polar molecules are barely affected by the buffer gradient. Retention times were again very consistent for each reequilibration time. Both phases showed a slight drift downwards in retention time as reequilibration time increased. Peak shapes and selectivity were also highly consistent. For both the polar and nonpolar bases, retention increases as reequilibration time is extended. For each set of basic probes, the magnitude of the increase on the pentahydroxy phase is greatly attenuated as compared to the bare silica phase. The hydrophobic probes show a greater variation in retention as a function of reequilibration time on the bare silica phase, consistent with the previous observations in the aqueous–organic gradients. This greater variation in retention supports the premise that relatively slow equilibration of the system is impacted by the ion-exchange mechanism and that the observed ion-exchange mechanism may be dependent on the slow adsorption of water on the surface. It is important to note that reproducibility at any given reequilibration time, on both phases, and with each set of probes, was again observed.
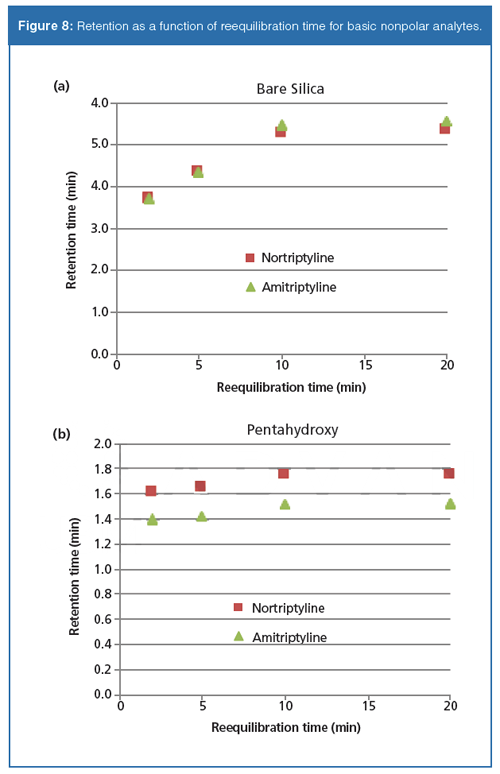
Conclusions
It was initially assumed that short reequilibration times in HILIC gradient methods would result in irreproducible chromatography. In addition, it was presumed that running gradients well outside the normal HILIC range would require additional reequilibration time to provide repeatable retention. Interestingly, the present study shows that consistent reequilibration times yield reproducible retention, selectivity, and peak shape. However, variability is observed as a function of reequilibration times, especially at extended times for basic analytes on the bare silica stationary phase. Each of the experiments hints that the presence of ion-exchange mechanisms results in slower overall equilibration and that the observed change in ionâexchange character may be the result of slow adsorption of water on the bare silica phase. The use of more polar HILIC stationary phases that exhibit less ion-exchange character may provide additional control of retention times when considering ionic analytes.
During the development of reversedâphase methods it is often assumed that if one allows the system to equilibrate for an extensive amount of time, the results would be the same as if the system were allowed to equilibrate for “just enough” time, typically equivalent to about 5–10 column volumes. The results of this study show that a gradient method in HILIC mode may appear equilibrated by virtue of reproducible retention times; however, with extended reequilibration time, the system is prone to change. It is therefore important that gradient HILIC methods are developed with reequilibration times in mind and that this parameter is specified in the final protocol to ensure reproducible and reliable results.
Reference
- D.S. Bell, LCGC Asia Pacific18(4), 26–32 (2015).
Daniel L. Shollenberger is a Senior R&D Scientist in the HPLC Surface and Design group at MilliporeSigma, a business of MerckKGaA, Darmstadt, Germany. He has spent nearly 10 years in the chromatography industry designing particle synthesis techniques, silane coupling reactions, and sampling devices. His current research focus is the application of existing and novel stationary phase chemistries to new generations of HPLC particle technology. Dan received a Masters in chemistry from Lehigh University specializing in analytical chemistry in the pharmaceutical sciences. He previously served as a chemistry instructor at Penn State University. He has a wide range of experience with different analytical techniques including sample preparation, gas chromatography, liquid chromatography, and mass spectrometry.
David S. Bell is a Senior R&D Manager at MilliporeSigma (formerly Sigma-Aldrich/Supelco). With a B.S. degree from SUNY Plattsburgh and a Ph.D. in analytical chemistry from The Pennsylvania State University, Dave spent the first decade of his career within the pharmaceutical industry performing analytical method development using various forms of chromatography and electrophoresis. During the past 15 years, working directly in the chromatography industry, Dave has focused his efforts on the design, development, and application of stationary phases for use in HPLC and hyphenated techniques. In his current role at MilliporeSigma, Dr. Bell’s main focus has been to research, publish, and present on the topic of molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. Direct correspondence to: LCGCedit@ubm.com

Discovering the Hidden Plastic in Garden Compost with Pyr-GC–MS
May 12th 2025An Australian study used pyrolysis coupled to gas chromatography-mass spectrometry (Pyr-GC–MS) to analyze the presence of plastic polymers in commercial and homemade composts. LCGC International spoke to Simran Kaur—a PhD candidate at the Queensland Alliance for Environmental Health Sciences (QAEHS) at The University of Queensland in Woolloongabba, Australia—to find out more about her team’s findings.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








