Investigation of Pore Size Particle Size and Column Geometry Effects on SEC Analysis of Monoclonal Antibodies
Special Issues
Smaller particle size stationary phases have seen increased use in the modern analytical laboratory due to their ability to exhibit greater resolution while enabling the use of smaller column geometries. Shorter column lengths and narrower bores allow for faster run times and lower flow rates, decreasing laboratory costs in terms of solvent consumption and man-hours needed to run the analyses. This application note examines the effects of smaller particle sizes in various column geometries on size-exclusion chromatography (SEC) methods and the method variables affecting the characterization of antibody products.
Smaller particle size stationary phases have seen increased use in the modern analytical laboratory due to their ability to exhibit greater resolution while enabling the use of smaller column geometries. Shorter column lengths and narrower bores allow for faster run times and lower flow rates, decreasing laboratory costs in terms of solvent consumption and man-hours needed to run the analyses. This application note examines the effects of smaller particle sizes in various column geometries on size-exclusion chromatography (SEC) methods and the method variables affecting the characterization of antibody products.

This work focused on scaling-down a monoclonal antibody SEC method, starting with a typical analytical-size SEC column (YMC-Pack Diol 5 µm, 300 Å, 300 ??8.0 mm) and ending with a small particle, shorter length, narrower-bore column (YMC-pack Diol 2 µm, 300 Å, 150 ??4.6 mm). The purpose of this experiment was to detail the differing performance characteristics exhibited by each column as physical properties were altered, one variable at a time.

Operating Parameters
Sample: 1 mg/mL Avastin in PBS
Mobile Phase: 100 mM sodium phosphate with
200 mM sodium chloride at pH=7.0
Column Temp: 25 °C
Flowrate: 1.0 mL/min for 8.0 mm I.D columns
0.33 mL/min for 4.6 mm I.D. columns
Inj. Volume: 30 µL for 8.0 mm I.D. columns
10 µL for 4.6 mm I.D. columns
HPLC System: Agilent 1260
Changes in column geometry were addressed by scaling flow rate (1.0 mL/min to 0.33 mL/min) and injection volume (30 µL to 10 µL) accordingly as inner diameter was decreased from 8.0 mm to 4.6 mm. This allows for a method that is typically run at 1.0 mL/min for 15 min to be run at 0.33 mL/min for 10 min, cutting run time by 1/2 and solvent consumption by a factor of 6x. For resolution comparability see Table I.
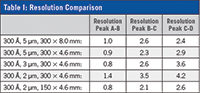
As the results indicate, when applied to SEC analysis of monoclonal antibodies, smaller particle stationary phases in shorter columns can increase throughput, while still providing adequate resolution that rivals the standard 300 ? 8.0 mm, 5 µm columns.

YMC America, Inc.
941 Marcon Blvd., Suite 201, Allentown, PA 18109
tel. (610) 266-8650, fax (610) 266-8652
Website: ymcamerica.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis














