Influence of Sample Solvent Composition for SFC Separations
LCGC North America
A discussion of different sample solvent compositions to be injected for SFC as part of injection linearity experiments
Significant peak distortion caused by sample diluent solvents occurs in all modes of chromatography. The condition known as "the strong solvent effect" can be very intense when the eluent strength of the sample solvent is much greater than that of the mobile phase. Usually, it is a good laboratory practice to dissolve the sample in the mobile phase into which the sample is being injected. For supercritical fluid chromatography (SFC) separations, doing so is close to impossible without extraordinary effort because the mobile phase is made up mostly of supercritical carbon dioxide. In this article, we consider several different sample solvent compositions to be injected for SFC as part of injection linearity experiments.
With the introduction of modern supercritical fluid chromatography (SFC) systems, a rapid increase of interest is expected. Many chromatographers new to SFC will be drawn toward the technique because of fast equilibration, short analysis times, and its limited use of greenhouse-producing solvents. For practitioners, transitioning from reversed-phase liquid chromatography (LC) separations will be made easier by the similarity between the software and physical components associated with modern systems. However, the use of supercritical carbon dioxide requires some special attention in a few areas. For example, what is the best way to prepare a sample for injection into an SFC system? Separations on low-dispersion SFC systems and sub-2-µm packing materials for SFC can be easily compromised by peak distortion. In this article, we demonstrate the effect of the sample solvent on peak profiles and make recommendations for selecting an appropriate solvent, as it pertains to SFC. For LC, the effects of sample solvents have been documented (1–6). Peak distortion has been reported in many cases, particularly for small molecules, and as a rule of thumb, samples should be prepared and injected in the mobile phase (or the initial mobile phase of a gradient) (7). Obviously, preparing a sample in a mixture of carbon dioxide and methanol, the most common mobile phase in SFC, is not a practical option. A diluent with a very low polarity is considered the best compromise when preparing a sample for injection in SFC. In the case where a solute is too polar to dissolve in a nonpolar solvent in detectable quantities, compromises must be made. Some specific suggestions regarding practices and compromises will be discussed later in this article.
Experimental
In this work, we compare the peak profiles of butylparaben dissolved in eight solvent systems. Injections of 0.3 mg/mL butylparaben in methanol, ethanol (denatured; ~4.9% isopropanol), isopropanol, tetrahydrofuran, 30:70 isopropanol–heptane, 50:50 tetrahydrofuran–heptane, and 30:70 tetrahydrofuran–heptane and dimethyl sulfoxide (DMSO). Peak area and height linearity are compared for each solvent system as well as chromatographic efficiency. The solvent systems were chosen based on elutropic properties on bare silica. Nonpolar solvents like heptane and hexane should be the best choices for diluents in normal phase–type separations (polar stationary phase, nonpolar mobile phase) (8). However, it should be noted that the use of volatile solvents will not only continuously concentrate samples in vials as the solvent evaporates, but also produce potentially hazardous vapors in the vial storage compartment. The best practices for an extended experiment are to cool the vial storage compartment well below room temperature and dispense the sample into multiple vials, especially when producing quantitative data.
Columns packed in-house with 5-µm Viridis Silica 2-Ethylpyridine (Silica 2-EP, Waters Corporation) and 5-µm Viridis BEH 2-Ethylpyridine (BEH 2-EP; Ethylene Bridged Hybrid bonded with 2-pyridylethyl ligand) into 150 mm × 2.1 mm hardware were used for this study. Injection linearity experiments were performed using a 5-µL sample loop installed on an Aquity UPC2 system (Waters Corporation), outfitted with a photodiode-array (PDA) detector. Injection volumes of 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, and 4.0 µL were used for each sample solvent. To match the retention factor of butylparaben on the two phases, two different mobile phases were used. A mobile phase of 95:5 carbon dioxide–methanol was used for Viridis Silica 2-EP and 97:3 carbon dioxide–methanol was used for Viridis BEH 2-EP. All separations were run under isocratic conditions and used a flow rate of 2.0 mL/min, a back pressure setting of 2000 psi and columns equilibrated at 40 °C. Detection was at 254 nm, compensation from 350 to 450 nm. Data were collected and processed using Waters Empower 3 software using the system suitability option.
Results and Discussion
When injecting a sample dissolved in pure DMSO or methanol, the peaks are always distorted when the injection volume is significant. DMSO and methanol were the most polar solvents investigated and therefore showed the most peak distortion. Even when our injection volume was just 0.5 µL, some slight peak distortion occurred. Often, users will find the peak distortion inconsequential or acceptable when very small injection volumes are used. Figures 1a and 1b show the peak height and peak area linearity for each solvent system on Silica 2-EP. Figures 2a and 2b show the same respective linearity for BEH 2-EP. The best performing solvent system under our injection tests was 3:7 tetrahydrofuran–heptane for both Silica 2-EP and BEH 2-EP.

Figure 1: Peak height (a) and peak area (b) linearity measured on a Viridis Silica 2-EP column using various sample solvent systems.
Up to 1.2 µg of butylparaben was injected using the best solvent tested here (3:7 tetrahydrofuran–heptane). To ensure that our data were not convoluted by mass overloading phenomena, more concentrated solutions of butylparaben were injected. We observed a linear detector response when injecting up to 15 µg of butylparaben on the column, until the detector was saturated (detection at 220 nm). Therefore, we were operating in the linear region of sample loading and the effects noted here are unrelated to mass overloading (9). For analytical separations, care should be taken to avoid overloaded peak profiles. Methods developed with high signal-to-noise detection allow practitioners to work in the linear region of adsorption isotherms because the samples injected are relatively dilute. All of our solvent systems tested had linear area responses, indicating that the injector was working properly for the experiment. Also, the best solvent system (3:7 tetrahydrofuran–heptane) produced a linear height response, confirming the sample was neither mass nor volume overloaded. Solvent effects and volume overload have been investigated by others (10,11). The degree of peak distortion is indeed related to the volume injected. In a sense, the peak distortion shown by the strong solvent effect is quite opposite to that of the "at-column dilution" technique (12,13). Eluites are concentrated at the inlet of a chromatographic column using a weak mobile phase and then are eluted with a stronger solvent. In our study, conditions were used such that volume overload is not a concern and the data is more clearly understood. Volume overload has characteristically wide chromatographic peaks and usually flat peak maxima. It should also be noted that the peak distortion is solute or solvent dependent, or more generally, retention factor dependent.

Figure 2: Plots of (a) peak height and (b) peak area linearity measured on a BEH 2-EP column using various sample solvent systems.
The correlation coefficients for each solvent system and injection volume are summarized in Table I. When the injection volume varied across a wide dynamic range (we were using 0.5–4 µL here), the experimental data revealed peak height linearity for solvents containing heptane. This can be considered a normal injection linearity experiment. If the peak area linearity is poor, low-level quantitation or instrumental issues are likely sources of the problem. We also considered the case of an injection linearity experiment designed with a smaller range of injection volumes in Table I (right-hand column). If the dynamic range is not large enough (for example, not including very small injection volumes compared to the sample loop volume), high correlations between injection volume and the peak area or peak height can be observed. If an analyst does not examine the actual chromatograms closely, he could accept the results as passing an injection linearity test. While the results are not wrong, as in our case where the injector was delivering the correct volumes of sample, they do not conform to the expected results. To avoid this possibility, we suggest practitioners use the normalized slope of the peak height versus injection volume as a guideline as to when peak distortion occurs for the sample in that particular diluent or solvent system. If the slope deviates significantly from unity, the data should be examined more closely. In Figure 3, we considered the effect of the peak distortion on the calculated peak efficiency. The same trends are noticed in that very polar sample solvents distort the peak profile, which significantly alters the calculated efficiency. Peak profiles for samples dissolved and injected in methanol, isopropanol, and 3:7 tetrahydrofuran–heptane are shown in Figure 4 on a BEH 2-EP column. An added benefit of mitigating peak distortion is increased detection and quantitation limits. The peaks are not only taller, but narrower, increasing the precision of peak integration. Viewing the injection linearity experiment chromatograms is a very quick and efficient way to understand the perils of peak distortion.

Figure 3: USP efficiency for (a) Viridis Silica 2-EP and (b) Viridis BEH 2-EP columns using various sample solvent systems. The most polar solvents distort the peak profile and reduce the calculated efficiency.
Examination of Table I reveals differences between the two columns tested here. The results are closely related to the fact that different mobile-phase compositions were used to match the retention factor of butylparaben on each phase. Two differences between the solvents injected into mobile phases of 95:5 and 97:3 carbon dioxide–methanol are at play in the results. First, when a sample dissolved in pure methanol is injected, the difference in local methanol concentration is greater in a 97:3 carbon dioxide–methanol mobile phase than a 95:5 carbon dioxide–methanol mobile phase. Second, the retention factor of methanol is significantly different between the mobile phases. Methanol from the injection plug will percolate through the column bed slower in the case of a 97:3 carbon dioxide–methanol mobile phase, increasing the local re-equilibration time. The latter effect is far greater for analytes with very low retention factors and grows negligible as retention factors increase. In our case, the retention factor is ~7 on either stationary phase, so the difference between local methanol concentrations greatly influences and distorts the peak profiles recorded.

Figure 4: Injection linearity chromatograms (BEH 2-EP; 97:3 carbon dioxideâmethanol) for butylparaben dissolved in 3:7 tetrahydrofuranâheptane (top), isopropanol (middle), and methanol (bottom). Injection volumes represented are: 0.5 (black), 1.0 (red), 1.5 (blue), 2.0 (green), 2.5 (light blue), 3.0 (pink), and 4.0 µL (brown).
In the past, it was recommended to purposely increase a chromatography system's dispersion between the injector and column inlet to reduce the peak distortion caused by injecting a strong solvent. The premise was that when more dispersion is present between the sample loop and column inlet, the sample solvent is more readily mixed with the mobile phase. Thus, the diluent solvent strength is weakened or diluted by the mobile phase. By using the mobile phase as the diluent, the injection volume is increased and the sample concentration is lowered. However, the injection band becomes wider, which can have a more significant consequence than that of the solvent distortion. To demonstrate this, the corrected peak efficiencies (13) were measured for a few compounds in reversed-phase LC using a C18 column (50 mm × 2.1 mm, 1.7-µm dp Acquity BEH C18 column, Waters). The ultrahigh-pressure liquid chromatography (UHPLC) system we used (Acquity H-Class UPLC, Waters) had about 14 µL of bandspreading (measured at the five sigma width, 0.35 mL/min, 65:35 acetonitrile–water, 25 °C) as typically configured. We intentionally added enough tubing between the injection valve and column inlet to raise the bandspread to about 63 µL (added 158 µL of volume). This is not a typical configuration and, in general, increasing the bandspreading for a chromatograph is unadvised. Two mixtures of acetone, naphthalene, and acenaphthene were injected under each system configuration. The first mixture was dissolved in 50:50 acetonitrile–water and the second was dissolved in pure acetonitrile. The results from this experiment are summarized in Table II. Under both system configurations, injecting the sample dissolved in pure acetonitrile produced a peak of lower chromatographic efficiency than the sample dissolved in 50:50 acetonitrile–water. That result is completely expected. The difference in efficiency between the two injections was about 11.5% on either system configuration, meaning the effect of diluent induced peak distortion was nearly the same. Injecting the same samples under the conditions of very high bandspreading, a markedly lower efficiency was recorded (by a factor of ~4). Using bandspreading and system migration time corrections, the results show very large increases in efficiency for the high-bandspread configuration, as expected. In the high-bandspread configuration, efficiencies increased 187% and 82% for the 50:50 acetonitrile–water and pure acetonitrile samples, respectively. In the case of the low-bandspread configuration, efficiencies increased 17% and 14% for the 50:50 acetonitrile–water and pure acetonitrile samples, respectively.
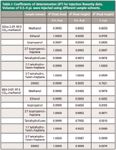
Table I: Coefficients of determination (R2) for injection linearity data. Volumes of 0.5â4 µL were injected using different sample solvents.
There are two very interesting results we found when adding bandspreading to a UHPLC system. First, in all cases we tested, the lowest-bandspread configuration gave us the highest efficiencies. This stresses the importance of operating an optimized system, containing low volumes and dispersion, relative to the column. Second, our data suggest that the efficiency loss because of peak distortion is nearly the same, regardless of the system bandspread. Because we observed an 11.5% and 11.8% drop in efficiency (uncorrected four sigma efficiency) when injecting a sample in pure acetonitrile on the high- and low-bandspread configurations, respectively, the biggest loss in efficiency was caused by injecting wider sample plugs from the high-bandspread configuration. In the high-bandspread configuration, the sample injection plug has much more time to mix with the mobile phase at its front and rear interfaces. The result is that the effective sample-plug volume increases and the concentration of strong solvent decreases. Ultimately, the results suggest that using a low-bandspreading system is of paramount importance. Although our example is an extreme case, the results are clear that dispersion has a large, deleterious effect on peak shape and efficiency. There may be some balance between additional dispersion and diluent induced peak distortion, but we suspect the effort necessary to define the conditions exceeds the desire of most users. Optimal results will be obtained when narrow, concentrated bands of sample are injected, in terms of efficiency and sensitivity. Furthermore, we suggest that practitioners should not attempt to mitigate diluent induced peak distortion by purposely adding dispersion to their chromatograph. The best option is to make changes to the method or sample solvent, which are summarized later. In our SFC experiments, we are operating the system in its typical configuration and consider it to be low dispersion, compared to similar chromatographs.

Table II: Efficiency changes caused by bandspreading correction and diluent induced peak distortion. Injection volumes were 1 µL into a mobile phase of 65:35 acetonitrileâwater.
Conclusions
There are a few practical considerations that can reduce peak distortion induced by the sample diluent. First and foremost, a sample should be dissolved in the weakest solvent (most nonpolar) that is compatible with the mobile phase. Very polar solvents such as DMSO and methanol will produce distorted peaks, even when small injection volumes are used. The lesser-retained analytes will be distorted the most. When polar solvents are necessary for sample solvation, blending with less-polar solvents should be considered. Increasing the strong eluent concentration in the mobile phase can be beneficial in terms of mitigating peak distortion, assuming the separation is not severely disrupted. In such a case, a balance between the required resolution and peak distortion mitigation can be made. Second, the sample injection volume should be made as small as possible, considering the injection reproducibility and detection limits of your system. As a consequence, it may be necessary to concentrate the analytes in the sample solvent to generate acceptable detector signals. In some cases, practitioners will need to concede some peak distortion to deliver their sample to the column in the desired concentration. For those situations, we do not recommend adding additional dispersion between the injector and column. Finally, low-bandspread instruments should be used — that is, those with minimal tubing and dispersion between the injection loop and column inlet. The improvement in efficiency from using a low-bandspread instrument is more closely linked to the fact that a narrower sample band is injected than to the strong solvent effect. Increasing the dispersion between the injector and the column inlet is not advised as a tool to mitigate peak distortion. Although the example discussed earlier is quite extreme, quadrupling the bandspreading on an instrument, the same general conclusions can be made for moderate increases in bandspreading.
In the case where a specific analyte cannot be dissolved in detectable quantities in a very weak solvent, a compromise must be made. If the analyte can be dissolved in relatively high concentrations in a stronger solvent, such as methanol, it should be done. Mixing the sample solution with a weaker solvent should be explored and applied when appropriate. The subsequent injection should be of the smallest applicable volume. The inherent risk of this procedure is precipitation of the sample after the injection plug is mixed with the mobile phase. Another alternative is to increase the amount of strong solvent in the mobile phase to reduce the polarity difference between the two fluids. Effectively, this is the result we are showing in the comparison between the two columns (Silica 2-EP and BEH 2-EP). So, if a method can benefit by increasing the amount of cosolvent, it should be increased accordingly. Obviously, the consequences for changing the mobile-phase composition will impact the resolution and pressure and those considerations should be examined on a case-by-case basis.
References
L.R. Snyder and J.W. Dolan, High-Performance Gradient Elution: The Practical Application of The Linear-Solvent-Strength Model, (Wiley-Interscience, Hoboken, New Jersey, 2007), pp. 190–193.
S. Keunchkarian, M. Reta, L. Romero, and C. Castells, J. Chromatogr. A 1119, 20–28 (2007).
K. Gedicke, D. Antos, and A. Seidel-Morgenstern, J. Chromatogr. A 1162, 62–73 (2002).
D. Vukmanic and M. Chiba, J. Chromatogr. 483, 189–196 (1989).
N. Hoffman, S. Pan, and A Rustum, J. Chromatogr. 465, 189–200 (1989).
P. Jandera and G. Guiochon, J. Chromatogr. A 588, 1–14 (1991).
U.D. Neue, HPLC Columns: Theory, Technology, and Practice, (Wiley-VCH, 1997), p. 355.
C.F. Poole, in The Essence of Chromatography, (Elsevier Science, 2002), p. 336.
G. Guiochon, A. Felinger, A. Katti, and D.G. Shirazi, Fundamentals of Preparative and Nonlinear Chromatography (Elsevier, 2006), pp. 13–14.
M. Zapata and J.L. Garrido, Chromatographia 31, 589–594 (1991).
J. Layne, T. Farcas, I Rustamov, and F. Ahmed, J. Chromatogr. A 913, 233–242 (2001).
H. Claessens and M. Kuyken, Chromatographia 23, 331–336 (1987).
U.D. Neue, C.B. Mazza, J.Y. Cavanaugh, Z. Lu, and T.E. Wheat, Chromatographia Supplement 57, S121–S127 (2003).
D. Guillarme, S. Heinisch, and J.L. Rocca, J. Chromatogr. A 1052, 39–51 (2004).
Jacob N. Fairchild is a senior research chemist at Waters Corporation in Milford, Massachusetts. Jason F. Hill is a research chemist at Waters Corporation. Pamela C. Iraneta is the evaluation group manager at Waters Corporation. Direct correspondence to: jacob_fairchild@waters.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).