Increasing LC–MS–MS Sensitivity with Luna HILIC
The Application Notebook
The analysis of polar compounds in support of clinical and pre-clinical pharmacokinetic studies requires an analytical methodology capable of achieving ultra-low detection and quantification limits. The high sensitivity afforded by coupling HPLC with tandem mass spectrometry (MS–MS) has made it the technique of choice in this environment, but it is subject to the following limitations when reversed phase liquid chromatography (RPLC) is used
A. Carl Sanchez, Monika M. Kansal, Art Dixon, Philip J. Koerner and Terrell Mathews, Phenomenex Inc., Torrance, California, USA.
The analysis of polar compounds in support of clinical and pre-clinical pharmacokinetic studies requires an analytical methodology capable of achieving ultra-low detection and quantification limits. The high sensitivity afforded by coupling HPLC with tandem mass spectrometry (MS–MS) has made it the technique of choice in this environment, but it is subject to the following limitations when reversed phase liquid chromatography (RPLC) is used:
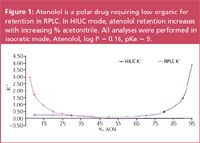
Figure 1
1. Polar compound elution in a highly aqueous mobile phase: Gas phase ion generation is facilitated with more volatile solvents;1 therefore, the high water content used for RPLC retention of polar compounds decreases desolvation efficiency. In addition the highly aqueous mobile phase required for retention of polar compounds increases solvent surface tension, which decreases ESI spray stability.2
2. Polar compound elution in the primary ion suppression region: Poorly retained compounds in RPLC increase the likelihood of analyte compounds eluting with the many endogenous compounds present in bioanalytical samples. These endogenous compounds compete for charge in the source and can suppress analyte ionization, reducing sensitivity and degrading LOD and LOQ. It is critical that a robust bioanalytical method separate analytes from the ion suppression region.3
Luna HILIC columns allow for improved sensitivity in the analysis of complex bioanalytical samples by creating more favourable conditions for polar compound retention and ionization.
Experiment
On the Luna HILIC column, atenolol in a high organic volatile mobile phase, which facilitates analyte desolvation and results in enhanced LC–MS–MS sensitivity.

Figure 2
Polar metabolites are weakly retained under RPLC conditions and elute near the solvent front, often in the "primary ion suppression region".
Under HILIC chromatographic conditions, where polar analytes are retained using high organic mobile phase, Luna HILIC strongly retains polar compounds that are otherwise weakly retained in RPLC.
Conclusion
The Luna HILIC column retains and elutes hydrophilic/polar compounds in highly organic mobile phase conditions, which improve analyte desolvation efficiency and ESI spray stability. The combination of these two improvements in the MS interface will often provide improved polar analyte detection and quantification.
In HILIC mode, analyte elution in the primary ion suppression region is avoided as a result of the mode's increased retention of hydrophilic compounds. This increased retention for polar compounds regularly results in less interference from endogenous sample compounds, improved sensitivity and accuracy, and reduced LOD and LOQ. The Luna HILIC column meets the requirements of a robust bioanalytical method by eluting analytes outside the primary ion suppression region.
It has been shown here that Luna HILIC columns can improve LC–MS–MS sensitivity of polar compounds in biological matrices in support of clinical and preclinical pharmacokinetic studies where ultra-low detection and quantification limits are highly desirable.
References
1. N.B. Cech, C.G. Enke, Mass Spectrom. Rev., 20, 362–387 (2001).
2. P.J. Kerbarle, J. Mass. Spectrom., 35, 804–817 (2000).
3. B.K. Matuszewski, M.L. Constanzer and C.M. Chavez-Eng, Anal. Chem., 75, 3019–3030 (2003).

Phenomenex Inc.
411 Madrid Avenue, Torrance, California 90501, USA
tel. +1 310 212 0555 fax +1 310 328 7768
E-mail: info@phenomenex.com
Website: www.phenomenex.com

A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.
The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.






