Identifying Drugs of Abuse Using Gas Chromatography-Time of Flight Mass Spectrometry (GC-TOFMS)
The Application Notebook
Illegal drug use worldwide is at an all time high. Forensic laboratories are seeing increased sample loads creating an immediate need for fast and accurate analysis to positively identify confiscated materials in criminal investigations. This application highlights the value of gas chromatography with time-of-flight mass spectrometry (GC-TOFMS) for drug testing in forensic laboratories. A method was developed to successfully identify twenty drugs of abuse in 4.5 min. This GC-TOFMS method shows good chromatographic peak shape for even the most challenging drug analytes; even the peak shapes for amphetamine and methamphetamine were exceptional considering they were analyzed underivatized. The total ion chromatogram (TIC) for the twenty drug analytes is shown in Figure 1.
Illegal drug use worldwide is at an all time high. Forensic laboratories are seeing increased sample loads creating an immediate need for fast and accurate analysis to positively identify confiscated materials in criminal investigations. This application highlights the value of gas chromatography with time-of-flight mass spectrometry (GC-TOFMS) for drug testing in forensic laboratories. A method was developed to successfully identify twenty drugs of abuse in 4.5 min. This GC-TOFMS method shows good chromatographic peak shape for even the most challenging drug analytes; even the peak shapes for amphetamine and methamphetamine were exceptional considering they were analyzed underivatized. The total ion chromatogram (TIC) for the twenty drug analytes is shown in Figure 1.
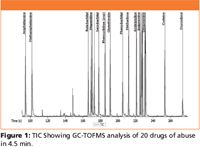
Figure 1
This method was then used to analyze real world samples obtained from a collaborating forensic laboratory. The samples were residues collected by cotton swab that were taken at a suspected drug house.
Experimental
GC: Agilent 7890
Column: Phenomenex ZB-DRG1
Oven: 70 °C to 280 °C, hold 1.5 min
Injection: 2 μL, splitless at 250 °C
Carrier: He, ramped 0.43 to 2.0 mL/min
MS: LECO TruTOF HT
Acquired mass range: 30–500 m/z
Acquisition rate: 10 spectra/s
Source temperature: 300 °C
Samples
A standard containing twenty drugs of abuse was obtained from Alltech. This standard was analyzed by GC-TOFMS and the ChromaTOF software was used to create a reference containing both mass spectral and retention time criteria for each analyte.
Cotton swabs collected at various locations within the suspected drug house were extracted and resulting extracts were analyzed against the reference.
Results and Conclusions
The reference created using the ChromaTOF software was used to compare unknown materials against mass spectra and retention times of known drug substances.
The results showed the presence of cocaine on all cotton swabs collected at the drug house. Figure 2 shows overlaid extracted ion chromatograms (EIC) for the cocaine peak in both the reference standard and Swab #1. Spectra for the swab and cocaine reference standard and the peak table showing a match for cocaine are inset in the figure.

Figure 2
The use of the LECO TruTOF GC-TOFMS and ChromaTOF software offers significant advantages for analysis and positive identification of drug substances.
One major benefit of utilizing TOFMS for drug identification is the ability to acquire full mass range spectra without sacrificing sensitivity. This is essential for positive identification of materials that may be present at trace levels. This work has demonstrated the ability of GC-TOFMS to increase laboratory productivity and efficiency while providing positive identifications of illegal drug substances in criminal investigations.

LECO Corporation
3000 Lakeview Avenue, St. Joseph, MI 49085
tel. (269)985-5496, fax (269)982-8977
Website: www.leco.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)