HPLC Autosamplers: Perspectives, Principles, and Practices
LCGC North America
We explain recent trends in HPLC autosampler design, provide recommendations for selecting one, and offer guidelines for operation and troubleshooting.
This installment is the second of a series of four white papers on high-performance liquid chromatography (HPLC) modules focusing on pumps, autosamplers, ultraviolet (UV) detectors, and chromatography data systems, respectively. This installment provides a technical overview of HPLC autosampler design, historical perspectives, and recent trends. Recommendations for selection of autosamplers and guidelines for operating and troubleshooting are provided.
The autosampler, an automated sample injector, introduces a precise aliquot of a sample solution from a sample container to the high performance liquid chromatography (HPLC) column under high-pressure flow conditions. It is a sophisticated automation device, capable of great precision and long-term reliability. The historical evolution of manual injectors and auto-samplers have been reviewed in many textbooks (1–4) and journal articles (5). Their performance, pressure ratings, and reliability have increased dramatically in the last two decades to accommodate demands from the use of narrower columns in ultrahigh-pressure liquid chromatography (UHPLC) (6–9). In this article, an overview of HPLC autosamplers is provided by describing their requirements, historical perspectives, modern design and trends, operating principles, sampling configurations, and common operating, maintenance, and troubleshooting procedures. The focus here is on analytical-scale applications, which require high-precision analysis in regulated laboratories.
Glossary of Key Terms and Definitions
- Autosampler: A device that automates the introduction of an aliquot of sample solution to the HPLC column. An HPLC autosampler typically comprises a sample storage compartment with an injector, consisting of a valve, a sample dosing or metering device, and a moving sampling needle.
- Injection Valve: A device such as a rotary valve that allows the introduction of a sample solution in the HPLC column under high-pressure and flow conditions. An injection valve can be a standalone manual valve, or part of an autosampler. It typically consists of a needle port, a rotor and stator combination, and an exchangeable sample loop.
- Pulled-loop, pushed-loop, or split-loop design: These are the three common modes of sample introduction designs. A split-loop design includes the sampling needle as part of the sample loop, and aspires an exact sample aliquot to be introduced. A pulled-loop design aspires the sample by vacuum from an external sampling syringe into the loop. A pushed-loop design fills a loop with pressure.
- Full-loop or partial-loop mode: Two modes of injection are used with pulled- or pushed-loop autosamplers. In a full-loop (or fixed-loop) mode, the entire sample loop is filled with the sample solution. In a partial-loop or variable-loop mode, only a fraction of the loop (10%–50%) is filled. The full-loop mode is more precise, particularly for small-volume injections, but requires more sample solution to overfill the loop (three times the sample loop volume to ensure that the entire loop is filled with the sample solution). The partial-loop mode offers more flexibility than the full-loop mode by its ability to inject different sample volumes without a physical change of the sample loop.
- Sampling syringe or metering device: A precise volume dosing unit required for sample aspiration. Two types are commonly used. One is a flow-through metering device, which is a part of the high-pressure flow path of a split-loop autosampler; the other is a low-pressure dead-end sampling syringe.
- Sample container: Typically, a 2-mL glass vial containing the sample dissolved in a diluent (final sample solution). Vials are placed in an autosampler-compatible vial holder, typically rectangular vial trays (vial racks), or vial carousels. Other configurations are microplates, commonly used in high-throughput screening (HTS) applications.
- Sampling needle: Typically, a 22-gauge blunt-tip needle of a manual sample syringe connected to the end of a movable stainless steel or polyether ether ketone (PEEK) sampling capillary, typically used with pushed-loop type. Split-loop autosamplers use highly specific sampling needles that match the needle-port, and must be capable of operating at high pressure.
- Sample compartment: An enclosed compartment for storing sample containers, which is commonly thermostated. An optional device (plate changer) can be used to transport additional sample trays or microplates, and increase sample capacity.
- Needle wash port: A needle port connected to waste for parking the sampling needle and rinsing the outside of the sampling needle with a solvent that is supplied by a peristaltic pump (or by the sampling syringe flushing through the inside and outside of the needle). The needle wash is used to reduce carryover from the previous sample, which adheres to the outside of the needle.
- Needle seat/port: For pushed-loop autosamplers, an orifice through which the sampling needle can fill the sample loop; for split-loop autosamplers, a counterpart port for the sample needle that must seal to the maximum system pressure level. This port is not required for pulled-loop autosamplers.
- Carryover: The amount of the main component from the prior injected sample, which is observed in a subsequent injection of the sample diluent (blank) without analyte. Carryover performance is highly analyte-dependent, and is particularly problematic for very basic compounds and proteins.
- Injection volume precision: Precision reflects the variation (scatter) of the delivered sample volumes during repetitive injections as a relative standard deviation (RSD) of peak area or peak height.
- Injection volume accuracy: Accuracy describes the deviation or systematic error of the absolute injection volume from the intended volume. The volume accuracy requirement is less relevant in practice, since method calibration (standardization with a reference compound) is performed in quantitative HPLC analysis.
- Injection cycle time: The time required to accomplish a sample injection that includes device movements to bring the sampling needle to the sample, sample volume aspiration, and needle wash or loop cleaning. The injection cycle can be reduced by overlapping with the ongoing separation cycle time (if allowed by the instrument and control software).
- Pressure transducer: The pressure sensor in the autosampler flow path used in some autosamplers to monitor the injection sequence. The pressure sensor can be used to optimize the injection routine timing, or warn the customer in case of any irregular behavior.
Solvents used in the autosampler include:
- Wash solvent: the rinsing liquid(s) used to clean the sampling needle and loop to reduce carryover.
- Carrier solvent: the liquid used to aspire the sample into the sampling needle, needle capillary, and sample loop, typically in direct contact with the sample solvent. The mobile phase can also be used as a flush or carrier solvent in some designs (the flow-through metering device in split-loop samplers).
- Diluent: the solvent for diluting the sample in the final sample solution, which should be weaker than the initial mobile phase, to avoid potential peak distortions. Note that the sample injection volume is proportional to the column void volume (3).
Requirements and Characteristics
Table I summarizes the requirements and desirable characteristics of a modern HPLC injector or autosampler. What follows below are discussions of historical perspectives, injector designs, operating principles, and sampling configurations. Our goal is to increase the understanding of the HPLC autosampler to the laboratory scientist, thus allowing the implementation of better operating practices or purchasing decisions.
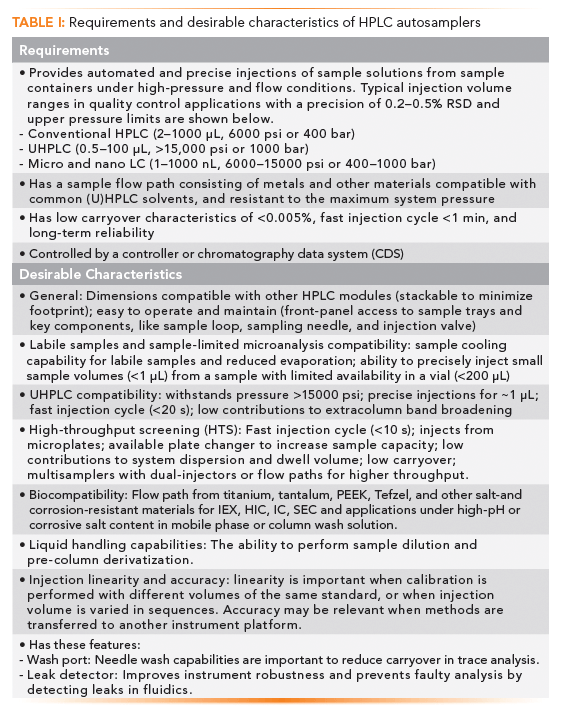
Besides obvious requirements such as the ability to withstand high pressure (6000 psi to 22,000 psi or 400 to 1500 bar) and compatibility to all common HPLC solvents and mobile phase additives, the most important requirement is sampling volume precision (<0.5% RSD for regulatory analysis) and flexibility of sampling volume (2–1000 µL) (3). Low carryover is important for critical assays such as bioanalytical or multi-residue assays, and purity analysis, while a fast injection cycle time is important for high-throughput applications. For many biopharmaceutical applications using ion-exchange chromatography, hydrophobic interaction chromatography (HIC), or size exclusion chromatography (SEC) separation modes that use mobile phases with high-salt content and column rinsing with basic eluents (>pH 10), the use of "biocompatible" materials such as titanium, Ni-Cr-Co alloy (MP35N), and base-resistant polymers in the fluidic pathway is highly recommended (3,7,9).
Note that sample volume accuracy and linearity are less important than precision performance in practice, because most quantitative HPLC analyses use a calibration process with a reference standard solution. Nevertheless, these attributes are often verified during HPLC system operational qualification in some pharmaceutical laboratories (4).
Other autosampler attributes, such as contributions to system dwell volumes or extracolumn dispersion, are less important in practice and become more relevant in highly optimized UHPLC methods. The contributions to dwell volumes from auto- samplers are usually insignificant in comparison to those from HPLC pumps (except for UHPLC binary pumps with small mixers) (8–10). Pre-column system dispersion is mostly important for isocratic analysis, due to the absence of the sample zone focusing at the column inlet (3,10). Most critical assays are conducted in (gradient) reversed-phase chromatography (stability-indicating analysis) where pre-column sample dispersion has little impact due to "peak compression effect" during gradient elution (1,3). The exceptions are for early-eluting peaks in gradient chromatography. Another important exception is in size-exclusion chromatography (SEC), using UHPLC columns where extracolumn band broadening can cause significant miscalculation of molecular weight averages for organic polymers (3), or in the determination of aggregates levels of monoclonal antibody (mAb) therapeutics (11).
Key Components
An autosampler comprises two major subunits: a sample injector and a sample compartment. The sample injector's role is to deliver a precise sample aliquot to the column, and typically consists of a sample metering device or sampling syringe, a rotary injector valve (or valve system), a sample loop, a sampling needle or capillary, and a needle port or seat (depending on design). The role of the sample compartment is to provide storage of a large number of sample solutions in sample containers (vials or microplates) under ambient or thermostated conditions to allow sequential injections and analysis. The sample containers are organized in sample trays with additional sample capacity provided by optional plate and vial tray changers (or chargers).
Brief Historical Perspectives
There are numerous historical accounts of HPLC and its instrumental development (1,2,5). Here are brief highlights on the development of different types of manual injectors and autosamplers leading to the modern renditions in use today.
Early Manual Injectors
Before the 1970s, injections were performed manually, using a microsyringe with a sharp needle through a septum, similar to the injection process in gas chromatography. The injection process was unreliable and the weakest link in the HPLC process, due to frequent leaks from the punctured septa under high pressures.
Waters U6K Septum-less Injector
The first reliable manual injector was a septum-less loop-based Model U6K injector introduced by Waters Corporation in 1973 (12). The U6K uses a set of manual switching valves, a sample loop, and a needle port, where samples are introduced into the loop under no-flow conditions. The needle port is then closed with a plug, and the sample is delivered into the flow stream by switching another valve, which also triggered a recorder chart spike for signifying the start of the chromatogram. While the U6K is cumbersome by today's standard, it was perhaps the only injector with adequate reliability until the advent of the rotary injector valve a few years later.
Rheodyne 7125 Manual Sample Injector
The Model 7125 manual sample injector introduced by the Rheodyne Corporation (acquired by IDEX in 2002) quickly became the industry-standard injector in the late 1970s (13). The compact injector consists of a two-position, six-port valve with a rotor, an interchangeable sample loop (6 µL to 2 mL), and a front-loading needle port (Figure 1a). The rotor is turned by a handle, and has a high-pressure rotor seal pressed between the valve body and a stator with six connection ports. A manual syringe with a 22-gauge blunt-tip needle is used to introduce a sample aliquot through the needle port connected to the sample loop during the loadposition (Figure 1b). This clever design with a built-in needle seat in the rotor seal coupled directly to the sample loop allows the entire sample aliquot to be transferred to the sample loop under atmospheric pressure. While the needle tip is still in the seat, the sample is delivered by switching the valve to the inject position (Figure 1c).
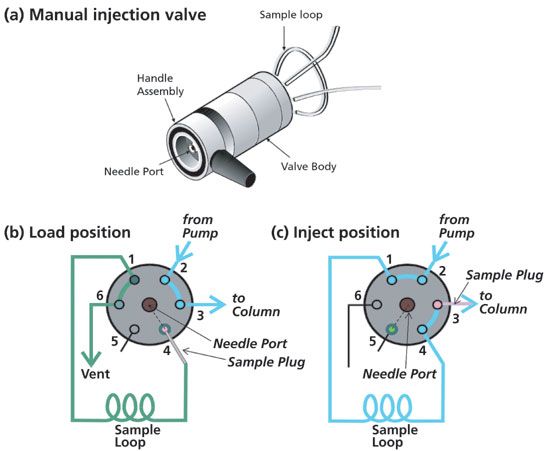
Figure 1: Schematic diagram of (a) the manual injection valve (Rheodyne model 7125); (b) schematic of the 7125 injector in Load position; (c) and in Inject position. Figures adapted from reference (3).
For best precision, the sample loop is overfilled with a minimum of three times the sample loop volume in a "full-loop" injection mode. A sample loop of a different volume is required to change sample volumes in this full- or fixed-loop mode. For better sampling volume flexibility, a partial-loop fill mode is used where different sample aliquots can be injected, as long as they do not exceed 70% of the sample loop volume for this manual injector. Note that the sample is back-flushed into the column to minimize band broadening in the sample loop (1,3). The 7125 injector was further refined into the model 7725, which incorporates a "make-before-break" bypass feature to prevent the momentary pressure cutoff during the injection process, which reduces column lifetime (14).
Early Autosamplers
The development of a reliable rotary injector led to the implementation of many early autosamplers. The first ones were simple devices using a rotary turntable containing filled sample vials and nitrogen pressure to overfill the sample loop of a remotely actuated injector valve. Other early autosamplers such as the PerkinElmer Series 200 autosampler, introduced in the 1990s and described elsewhere (3), mimic the manual injection process with a moving sampling needle, which can access up to 100 sample vials in a tray, and deliver the sample aliquot into a fixed-position 7125 injector valve that is remotely actuated by a contact closure.
Types of Autosamplers and Operating Principles
While all autosamplers consist of key components serving similar needs (injection valve, metering device, sampling needle, sample loop, and sample compartment), there are significant differences in the operating principles between various types of autosamplers. This section explains the functional differences between the three main injection principles, which are pushed-loop, pulled-loop, and split-loop injection modes. This section also describes the consequences of each injection principle in regard to engineering needs and benefits or limitations for the user. Additional discussions of the different types of injection approaches can be found elsewhere (9,15).
The Pushed-Loop Design
The principle of the pushed-loop design is perhaps closest to a manual injection process, utilizing a low-pressure sample needle port built into or external to the injection valve. This design contains two fluidic sections, as illustrated in Figure 2. The first section, called the aspiration capillary, is used for the initial sample aspiration and storage. While the injection valve is in its load position, the sampling needle moves to the sample and aspirates the sample aliquot by moving the plunger of the sampling syringe backward. The sample is now temporarily stored in the aspiration capillary. During that phase, the sample loop is bypassed and vented. In a second step, the needle moves to a low-pressure needle port. When inside the port, the sampling syringe pushes the sample aliquot into the sample loop. Finally, the injection valve is switched to the inject position.
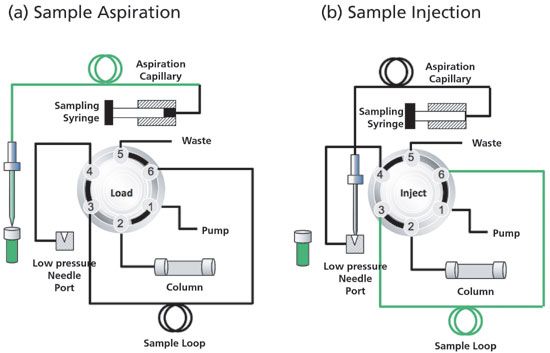
Figure 2: Schematic diagrams showing (a) the sample aspiration, and (b) the sample injection steps of the pushed-loop autosampler design.
A flush solvent is used to fill the metering syringe as a "carrier" solvent and provides a continuous fluidic path for sample withdrawals and delivery. Air gaps are often used to segment the sample plug from the carrier solvent. One example of the push-loop autosampler is the PerkinElmer Series 200, described elsewhere (3).
The Pulled-Loop Design
The pulled-loop design shown in Figure 3 is perhaps a simpler approach, because it does not require a needle port. Here, during sample aspiration, the needle dips into the sample liquid, and the sampling syringe directly draws the sample into the sample loop while the injection valve is in the load position. After the loop is filled (either partially or overfilled for full-loop operation), the injection valve can shift to its inject position, and the sample aliquot is delivered to the HPLC column.
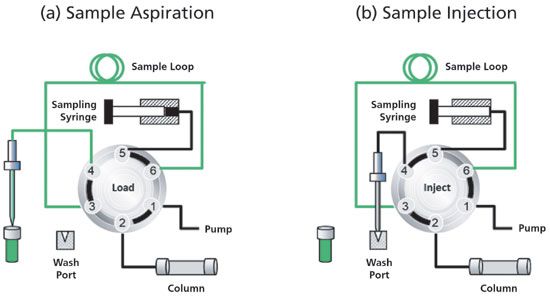
Figure 3: Schematic diagrams showing (a) the sample aspiration, and (b) the sample injection steps of the pulled-loop autosampler design.
Without the needle port, this setup is more robust from an engineering perspective, and has less contribution to pre-column dispersion and dwell volume. This sampler type has two main disadvantages. First, such design has a higher sample consumption, as not all aspirated sample is transferred to the column. In the full-loop injection mode, the consumption is often a factor of 3 higher than the injected sample. The second disadvantage is an increased carryover, which may be reduced with multiple rinsing of the sample loop with flush solvents (which negatively impacts the cycle time).
A modern example of the pulled-loop design is included in the Waters Acquity UPLC, which can operate in the "partial loop needle overfill" (PLNO) mode typically using two wash solvents (a weak and a strong solvent) and air-gap segmentations of the sample plug to maintain precision and carryover performance (16).
The Split-Loop Design
The most popular design in modern auto-samplers is the split-loop design (often called integrated-loop, flow-through needle, or needle-in-loop design). The split-loop autosamplers operate with the sampling needle as part of the sample loop. It has two major instrumental setups-with the metering device as part of the high-pressure flow path (Figure 4a), or placed outside of the high-pressure flow path (Figure 4b).
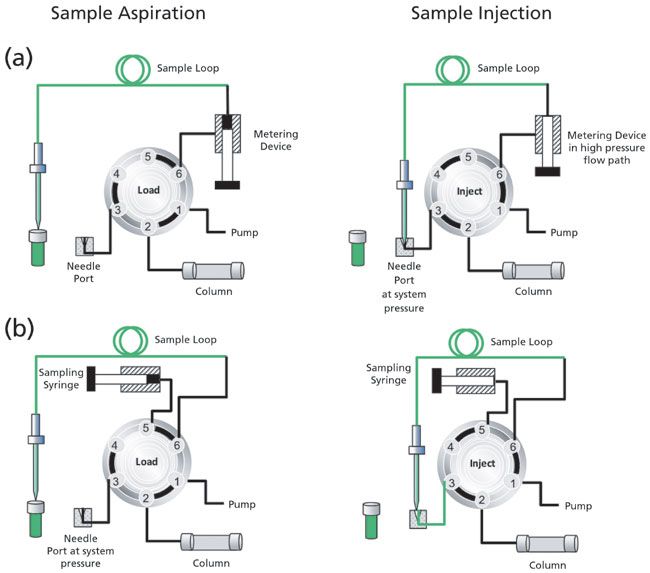
Figure 4: Illustration showing the sample aspiration and injection steps of the split-loop autosampler design: (a) Using a flow-through high-pressure metering device; and (b) using a dead-end sampling syringe.
In the split-loop design, the injection needle is directly connected with the sample loop with a high-pressure needle seal. During sample aspiration, the needle moves to the vial, and splits the loop into two portions. The sample aspiration can be done in two ways. The first option is to use a metering device in the mobile phase flow-path with the benefit of more precise injection and the constant flushing of the device, so that no air bubbles can accumulate (Figure 4a, such as in Thermo Scientific Vanquish and Agilent Infinity II 1290). Alternately, the sampling syringe can also be placed at a dead end without being flushed throughout the run (Figure 4b, such as in Thermo Scientific UltiMate 3000 and Waters I-Class FTN). However, special care must be taken that no air accumulates in the syringe, for instance, by using degassed liquids. The design has the advantage of reduced dwell volume for high-throughput screening (HTS) applications.
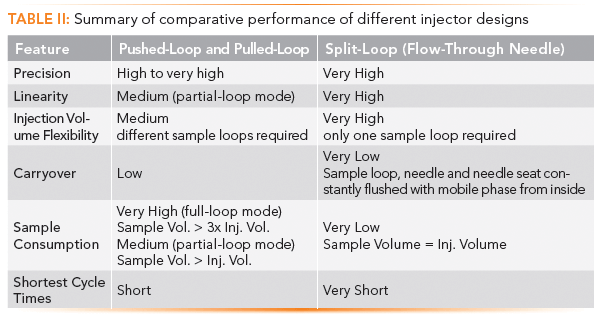
Comparative Performance of Different Injector Designs
Table II summarizes of the comparative performance of auto-samplers with different injector designs, indicating a strong superiority for the split-loop design, with better precision and carryover performance.
Autosampler Types Based on Sampling Configurations
Autosamplers can be categorized by sampling configurations such as the "vial-to-needle" or "needle-to-vial" approach, with additional considerations for the ability to handle microplates. These configurations are illustrated in Figure 5, and further discussed here.
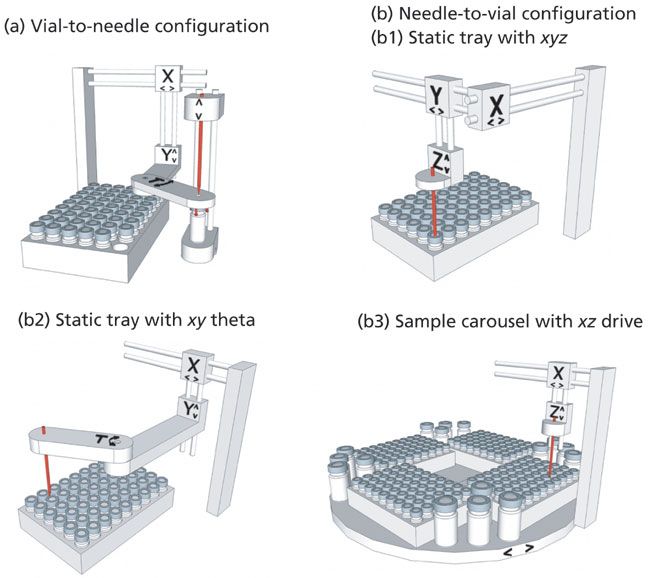
Figure 5: Diagrammatic illustrations of technical concepts of sample configuration: (a) vial-to-needle; (b) needle-to-vial; (b1) static tray with x-y-z drive; (b2) static tray with x-y theta drive; (b3) sample carousel with x-z drive.
The two major subunits of autosamplers are the sample compartment to host the sample containers or vessels, and the injection device, which also includes the flush and needle wash solvent flow paths. The actual design of each subunit depends on the technical implementation to bring the sample aliquot from the designated sample vial or microplate well to the injector. In this regard, there are two different implementations. One is the "vial-to-needle," and the other is the "needle-to-vial" approach, which can have a static tray or carousel format. For simplicity, we assume a split-loop or a pulled-loop injector in this discussion, though the description is relevant for all injector types.
Vial-to-Needle Autosamplers
Vial-to-needle autosamplers (Figure 5a) require a robotic gripper that captures the vial from the tray, and moves it below the injection needle, as exemplified in the Agilent 1100 Standard Autosampler or Agilent 1260 Infinity Standard Autosampler (but not the Agilent High Performance or Micro Well-Plate Autosamplers). For this (mostly split-loop) type of autosampler, the vial is placed on the needle seat, and is removed prior to the sampling needle moving down into the high-pressure needle seal (Figure 5a). With this design, the needle only has to perform short distance movement in z-direction. This design eases the mechanical stress on the high-pressure needle seal and the whole sample loop, and gives more flexibility for loop volumes and loop materials. At the same time, the gripper can shake the vial to ensure sample solution homogeneity. Nevertheless, the big disadvantages are the longer injection cycle times and incompatibility with microplates.
Needle-to-Vial Autosamplers with Static Sample Tray
This approach is a variant for microplate-compatible auto-samplers, where the sample containers in trays are stationary after they are placed in the compartment. Hence, the needle performs an x-y-z movement (Figure 5b1, for example, PerkinElmer Series 200 and Waters Acquity Classic Autosampler) (3); or an x-z-theta movement (Figure 5b2), for example., Agilent 1260 Infinity High Performance Autosampler) to approach the containers. In case of the x-y-z movement, the x-y travels of the sampling needle are provided by two screw drive mechanisms controlled by stepper motors, while the up and down motion (z-direction) is driven by another motor, that typically travels with the moving sampling needle.
The typical set-up of x-y-theta (figure 5b2) movement is a screw drive mechanism to move the rotating needle arm in the x-direction, a motor for rotating (theta) the arm that holds the sampling needle to substitute the movement in the y-direction), and a screw drive mechanism to move all these parts together with the needle in the x-direction (17,18). The static tray and injection valve configuration makes the best use of space in the sample compartment, so that the compartment can be more compact. Unlike the carrousel-based autosamplers, there is no moving sample tray in the sample compartment. The injection cycle time is shorter than with vial-to-needle types, but still not the shortest possible. Due to the rapid movement of the sampling needle, there can be more mechanical stress on the drive mechanism. Since the vials or microplates are static, the only way to homogenize the sample solution is to program the needle movement in the vial by sample aliquot withdrawal and release sequences.
Needle-to-Vial Autosamplers with Sample Carousels
This alternative concept employs a carousel that can host either vial trays or microplates (Figure 5b3, for example, Thermo Scientific UltiMate 3000 WPS, Thermo Scientific Vanquish Autosampler, and Waters Alliance). Since the carousel performs the y-movement with the sample containers, the needle drive movement is reduced to x-z-direction with the maximum distance from the needle seat to the closest to carousel axis position. The design restriction on the sample loop and the mechanical stress are less than with static tray. Due to the independent y-movement of the carousel, this type allows for the shortest cycle times while it still moves and shakes the sample containers for homogenization to a certain extent. The disadvantage to the carousel design is the inefficient usage of the compartment space in the carousel center, thus reducing the sample capacity and the efficiency in thermostatting.
General Comments on the Sample Trays and Injector Designs
Regarding the general design of the sample compartment, reliable cooling is an important functional aspect of this subunit. Cooling devices in most modern autosamplers are Peltier devices, but the way to perform sample cooling may differ. The typical lower temperature limit is around 4 °C, as lower temperatures may illicit issues associated with condensed moisture and ice formation. One operational principle is to cool the sample container in an air stream, which requires a well-enclosed compartment for best temperature control. The other is a contact-based cooling, using metal blocks (aluminum) as a temperature sink near the samples and allowing for a more open design. The closed compartment concept with ventilation has advantages regarding the reduced formation of condensed water or exposure to the laboratory atmosphere. The alternate mode of contact cooling has the advantages for faster cooling and easier insertion of last-minute vials or microplates.
Market Landscape
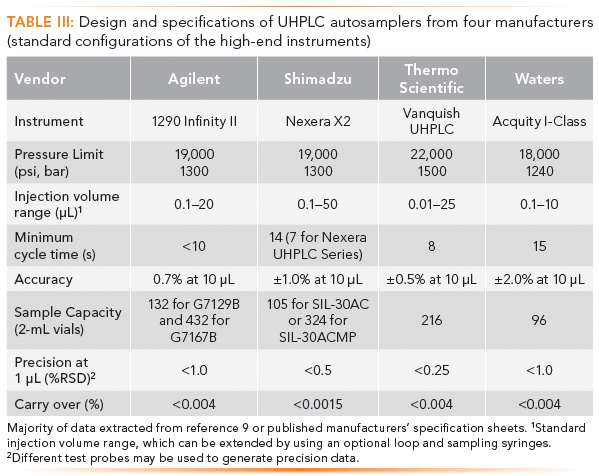
Table III is a summary of the design and specifications of modern UHPLC autosamplers from four major manufacturers (standard configuration of the high-end instruments). The information is extracted from a recent article (9), scientific literature, and updated manufacturers' specifications. Note that all four autosamplers employ the split-loop injection and needle-to-vial approaches, illustrating the modern trends of high-end HPLC autosamplers.
Case Study of Design and Performance of an UHPLC Autosampler (Thermo Scientific Vanquish)
There is a diversity of design types and technical implementations by different manufacturers, making it difficult to comment on their impacts on performance characteristics. To illustrate the design rationales and innovative implication, we will describe the technical details of one modern UHPLC autosampler as a case study (Thermo Scientific Vanquish) (17,18).
Introduced in 2014, the Vanquish autosampler has a default sample capacity of four trays hosted in a carousel (Figure 5b3), resulting in a total capacity of 216 for 2-mL vials. In addition, this autosampler can work with multiple vial sizes or standard microtiter plates, with barcode identification of different plate formats for added security. The trays are open to facilitate air circulation for efficient sample cooling. The injection mode is a split-loop design with a metering device in the high-pressure flow path and a fast x-z needle drive, while the carousel performs the movement in y-direction simultaneous to the x-drive. This design enables fast injection cycles down to 8 s with an injection peak area precision of <0.25% RSD for injection volume as low as 1 µL.
This autosampler uses SmartInject technology, which eliminates the pressure drop associated with the injector valve flow cut-off during rotation and improves the retention time precision significantly (by a factor of 6 in the example shown in Figure 6). In addition, the elimination of pressure shock improves column lifetime, and lessens potential interference with the analyte detection and quantitation of peaks near the solvent front.
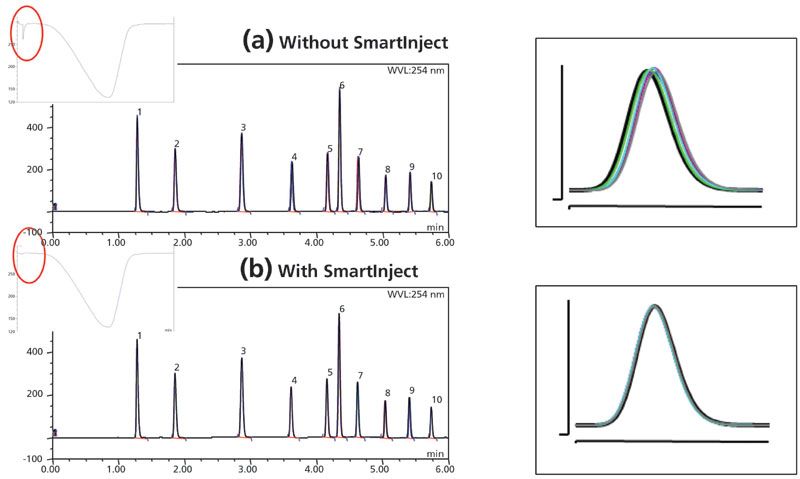
Figure 6: Effect of SmartInject technology on the elimination of pressure dips during the injection cycle and the resultant improvements of retention time precision showing (a) without, and (b) with, for an in average of six-fold improvement of precision for retention time.
Figure 7 shows another benefit of SmartInject technology during a size exclusion chromatography analysis using a multi-angle light scattering (MALS) detector for the aggregate analysis of a sample of bovine serum albumin (BSA) (11). The use of SmartInject eliminates a substantial interfering artifact peak caused by flow disruption associated with the injector valve rotation of a standard injection process.
A note worth mentioning is that the metering device used for the split-loop injection mode contributes to the system dwell volume. In general, that device has a flexible volume, and can thus be adjusted to change the overall dwell volume of a system. In this autosampler, this adjustment of dwell volume can be implemented in the chromatography data system (CDS), which can be helpful in method transfer scenarios where differences of dwell volume across different instrument platforms are sources of potential issues (10).
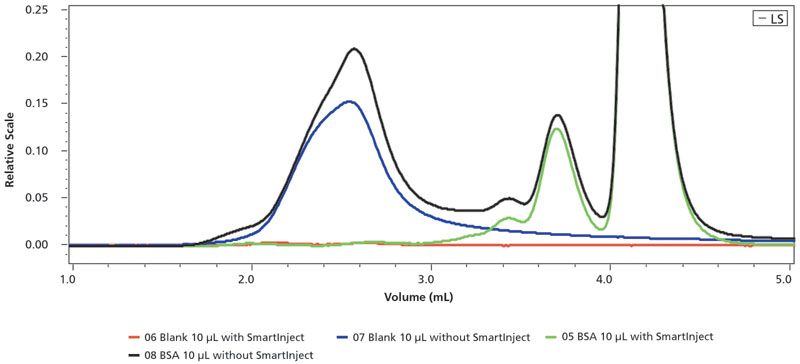
Figure 7: Size exclusion chromatography (SEC) using an autosampler with and without SmartInject Technology in combination with a Wyatt multi-angle light scattering (MALS) detector. The chromatograms illustrate the elimination of a significant artifact peak (first peak) of a bovine serum albumin (BSA) sample in the two lower chromatographic traces. The color key to the traces are as: (orange) 06 blank 10 µL with SmartInject, (blue) 07 blank 10 µL without SmartInject; (green) 05 BSA 10 µL with SmartInject, (black) 08 BSA 10 µL without SmartInject.
Finally, a unique dual split sampler is available to enhance laboratory productivity by providing a second injection flow path (each with its separate pump and detector) for performing two assays in parallel to double sample throughput as illustrated in Figure 8. Here, the same monoclonal antibody sample is characterized simultaneously by strong cation exchange chromatography for charge variant analysis and reversed-phase chromatography for intact protein analysis (11). This dual split sampler is capable of performing two entirely different assays at the same time.
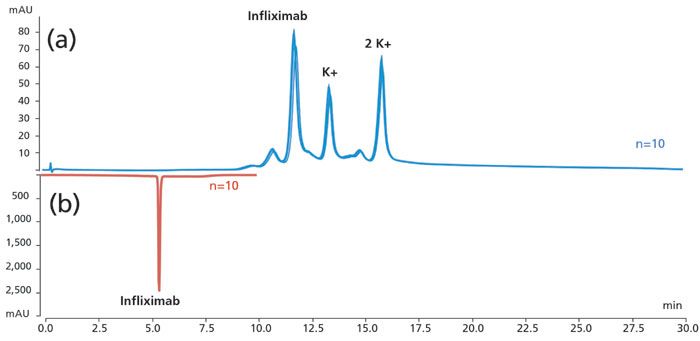
Figure 8: Simultaneous parallel characterization of a monoclonal antibody sample (Infliximab) by (a) strong cation exchange (SC-UV (with pH gradient) for charge variants, and (b) reversed-phase LC–MS (with acetonitrile gradient) for intact protein analysis, using a Thermo Scientific dual split sampler with two separate sample flow paths.
Modern Trends in Autosamplers
In recent years, HPLC autosamplers have become more sophisticated, with excellent reliability and performance. This is particularly obvious for newer UHPLC autosamplers with higher precision even for small-volume injections (<1 µL). Two application areas have seen the most recent progress in autosampler technologies: high-throughput screening (HTS) and automated sample preparation for complex sample matrices. Recent examples of new products designed for HTS include the Agilent Infinity II Multisampler, the Thermo Scientific Dual Split Sampler, and the Shimadzu Nexera UHPLC Series Auto-sampler, which can be equipped with up to three plate chargers capable of a total sample capacity of ~17,000 (19,20). Examples for automated sample preparation systems include LEAP PAL3 autosamplers (CTC Analytical) with customizable sample preparation modules and Shimadzu Clinical Laboratory Automation Module (CLAM) 2030 for blood analysis using triple-quadruple MS detection (20).
Other autosamplers designed for specific applications include Waters Alliance Dissolution System with a modified 2695 separation module capable of automated collection and injection dissolution samples in the sample carousels (21) and HTA's HT4000E SPE-LC sample preparation workstation for direct injections from solid-phase extraction cartridges.
Many autosamplers can perform additional simple liquid handling steps such as sample dilution, mixing, internal standard additions, and pre-column derivatization, though these procedures are infrequently performed, except for dedicated analyzers such as those for precolumn amino acid analysis (20). Another area of rapid development is the replacement of traditional materials in the most problem-prone key components, such as the rotor seal using carbon-reinforced polymer or ceramic, with lower friction and better wear properties (9).
Autosampler Selection, Operation, Maintenance, and Troubleshooting
Best practices in HPLC operation, maintenance, and troubleshooting have been described in books (3,22,23), journal articles (24,25), and manual or publications from manufacturers (26,27). A summary of practical guidelines for autosampler selection (for specific applications and options), daily operation, maintenance, and troubleshooting is included here as a general reference. Since these procedures are highly dependent on autosampler type and design, the reader is referred to manufacturers' manuals or resources on the specific model for details.
Selection Guidelines for Specific Applications and Optional Equipment
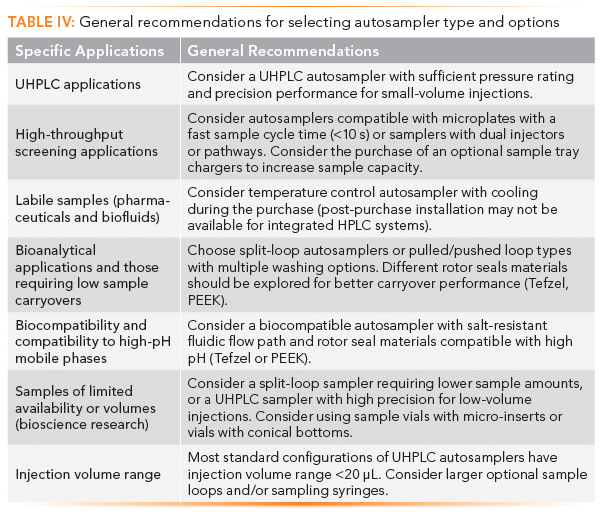
Table IV lists some of our general recommendations on autosampler types for specific applications and optional equipment. Note that the availability of software control drivers on the existing CDS for the specific HPLC system being considered for purchase may be an overriding consideration in most purchasing decisions.
Recommended General Practices for Autosampler Operation
Table V lists general recommendations for autosampler operation, including the selection of various autosampler-related solvents and sample diluents. The reader is referred to other publications for a more detailed recommendation of general best practices (3), including operating manuals or resources from manufacturers on specific autosampler models.
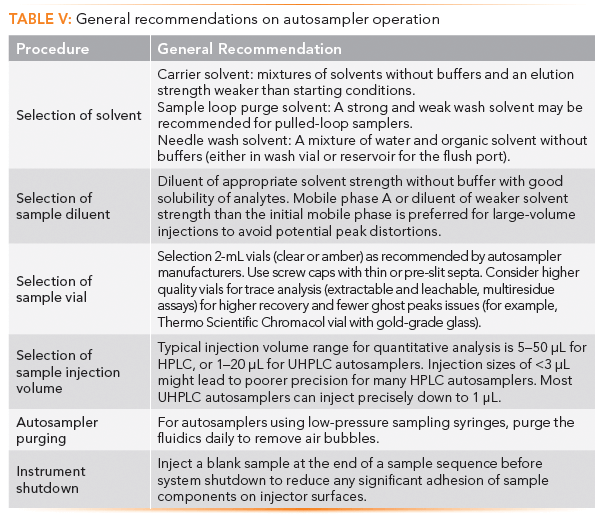
Autosampler Maintenance
The actual maintenance required is dependent on the type and design on the particular autosampler, and recommendations from the manufacturers should be followed. Some general maintenance comments of HPLC autosamplers are included here as a reference.
The reliability of HPLC injectors has increased significantly in recent years. The typical wearable item is the injector rotor seal for a rotary injection valve, which should be replaced periodically (annually) or when leaks occur. Under ideal conditions, a rotor seal can last 30,000 to 100,000 injections, though 10,000 to 20,000 injections may be more typical for many applications (13,15). This replacement for HPLC injector rotor seal can be accomplished by users with some practice following the manufacturers' instructions.
The sampling needle and the sampling syringe (low-pressure type) are also wearable items that require periodical replacement to restore sampling precision. An external sampling syringe with a Teflon (PTFE) tip is easily accessible and replaced by the user. The replacement procedure of the sampling needle or capillary can be more elaborate and might require a service specialist on some models.
For a split-loop autosampler, the high-pressure needle seat seal also requires annual replacement.
In regulated laboratories, all HPLC modules, including autosamplers, must be "calibrated" after annual preventive maintenance to maintain a "qualified" instrument status. The performance verification procedures and acceptance criteria are often similar to those found in operational qualification (4).
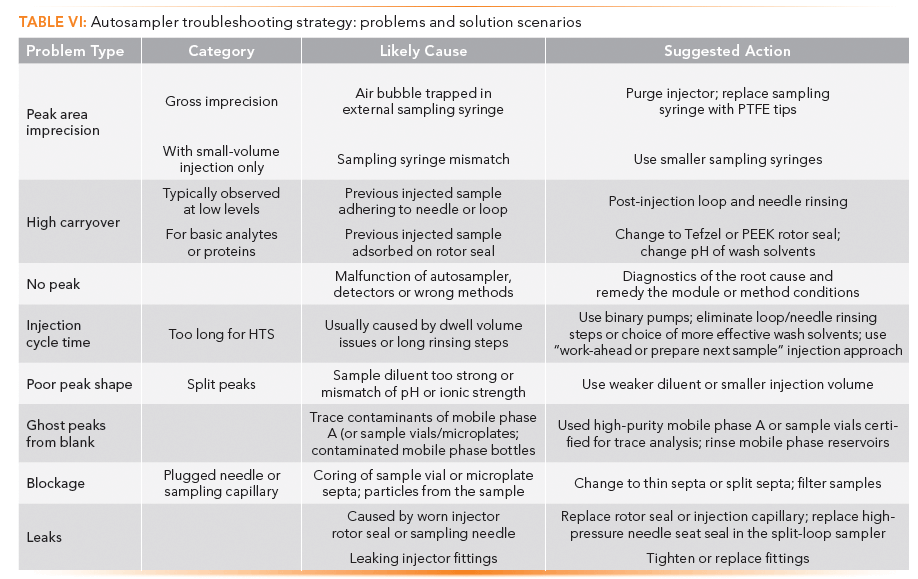
Autosampler Troubleshooting Strategies
The reader is referred to manufacturers' manuals and resources for details on troubleshooting specific autosampler models. In one case, an existing mobile app is available for a comprehensive troubleshooting guide for a particular UHPLC system (28). Note that some seemingly autosampler-related problems (imprecision of peak areas, no peaks, bad peak shapes) are often caused by other malfunctioning modules, columns, or method-related parameters (mobile phases, samples, injection volume, diluent, and so forth). Diagnosing problems may require a more holistic system approach (3). Common autosampler-related problems and solution scenarios, focusing on the performance or other issues such as peak area imprecision, carryover, cycle time, ghost peaks, leaks, or blockages, are listed in Table VI as a reference.
Summary and Conclusions
This installment provides an updated overview of the design of the injector and sampling approaches of modern autosamplers. The split-loop injector design with the "needle-to-vial" sampling configuration appears to dominate most high-end autosamplers because of its superior precision and carryover performance. Technical details and specifications of several autosamplers have been summarized, followed by a brief discussion of duo-path or dual-injector multisamplers for high-throughput screening, and others for automated sample preparation. General recommendations for autosampler selection and operation were described, together with guidelines on common maintenance and troubleshooting procedures.
Acknowledgments
The authors thank the following colleagues for their review of the manuscript: Ken Broeckhoven and Jelle De Vos of Vrije University of Brussel, Chris Pohl of Thermo Fisher Scientific, Davy Guillarme of University of Geneva, Mark Hayward of Stemline Therapeutics, Wulff Niedner of Bruker Daltonics, He Meng of Pfizer, Peter Ringeling of Spark Holland B.V., and Konstantin Shoykhet of Agilent Technologies.
References
(1) L.R. Snyder and J.J. Kirkland, Introduction to Modern Liquid Chromatography (John Wiley & Sons, Hoboken, New Jersey, 3rd ed., 2010), Chapter 3.
(2) L.R. Snyder and J. W. Dolan, Liquid Chromatography, S. Finali, P. Haddad, C. Poole, P. Schoenmakers and D. Lloyd, Eds. (Elsevier, Amsterdam, the Netherlands, 2013), Chapter 1.
(3) M. W. Dong, HPLC and UHPLC for Practicing Scientists, (John Wiley, & Sons Hoboken, New Jersey, 2nd ed., 2019), Chapters 2,4,5,7–9, and 11.
(4) S. Ahuja and M.W. Dong, Eds., Handbook of Pharmaceutical Analysis by HPLC (Elsevier/Academic Press, Amsterdam, the Netherlands, 2005), Chapters 11, 12, 15.
(5) C. H. Arnaud, Chem. Eng. News, 94(24), 29–35 (2016).
(6) D. Guillarme and M. W. Dong (Eds), Trends in Anal. Chem. (Special Issue) 63, 1–188 (2014).
(7) T. Fehrenbach and S. Wiese, The HPLC Expert II: Find and Optimize the Benefits of Your HPLC/UHPLC, S. Kromidas, Ed. (Wiley-VCH, Weinheim, Germany, 2017), pp. 223–267.
(8) M.W. Dong, LCGC North Am. 35(6), 374-381 (2017).
(9) J. De Vos, K. Broeckhoven and S. Eeltink, Anal. Chem. 88, 262 (2016).
(10) M.W. Dong, LCGC North Am. 35(11), 818-823 (2017).
(11) T. Zhang, C. Quan, and M.W. Dong, LCGC North Am. 32(10), 796–808 (2014).
(12) Model U6k Universal Liquid Chromatography Injector Instruction Manual, Waters, Milford Massachusetts, 1973.
(13) Operating Instructions Model 7125 Manual Sample Injector, Rheodyne LLC (a subsidiary of IDEX Corporation), Cotati, California, 2320157D, June 2001.
(14) J.L. DiCesare, M.W. Dong, and J.R. Gant. Chromatographia 15, 595–598 (1982).
(15) J. W. Dolan, LCGC North Am.34(5), 342-329 (2016).
(16) ACQUITY UPLC I-Class, System Specifications, Revision A, Waters Corporation, Milford, Massachusetts, 2011.
(17) Vanquish Split Samplers VH-A10, VF-A10, VH-A40, VF-A40 Operating Manual, 4828.5001-EN Revision 3.0, Germering, Germany, February 2018.
(18) Thermo Scientific Vanquish–Split Sampler HT/FT, Product Specifications, PS71198-EN 09/16M, Germering, Germany, 2016.
(19) M. W. Dong, LCGC North Am. 36(4), 256–265 (2018).
(20) M. W. Dong, LCGC North Am. 37(4), 252–259 (2019).
(21) Alliance HPLC Dissolution System, 720000251EN, Waters Corporation, Milford, Massachusetts, 2013.
(22) P.C. Sadek, Troubleshooting HPLC Systems: A Bench Manual (John Wiley & Sons, New York, New York, 2000).
(23) J. Dolan and L.R. Snyder, Troubleshooting LC Systems (Humana Press, Totowa, New Jersey, 1989).
(24) J. Dolan, "HPLC Troubleshooting," columns in LCGC North Am. 1983–2016.
(25) E. Grushka and I. Zamir, Chemical Analysis, Volume 98, P. Brown and R. Hartwick, Eds. (Wiley Interscience, New York, 1989), p. 529.
(26) U. Neue, HPLC Troubleshooting Guide, Waters Applications Notes, 720000181EN, Waters Corp., Milford, Massachusetts, 2002.
(27) Thermo Scientific Vanquish UHPLC System, Operating Manual, 4820–3601-En, Revision 3.0a, 2017, Thermo Fisher Scientific, Germering, Germany.
(28) https://play.google.com/store/apps/details?id=com.thermoscientific.troubleshootingguide&hl=de
ABOUT THE AUTHORS
Carsten Paul

Carsten Paul is a Product Marketing Manager for UHPLC products at Thermo Fisher Scientific, in Germering, Germany.
Frank Steiner

Frank Steiner is a Scientific Advisor at Thermo Fisher Scientific, in Germering, Germany.
ABOUT THE AUTHOR
Michael W. Dong

Michael W. Dong is a principal of MWD Consulting, which provides training and consulting services in HPLC and UHPLC, method improvements, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, Research Fellow at Purdue Pharma, and Senior Staff Scientist at Applied Biosystems/PerkinElmer. He holds a PhD in Analytical Chemistry from City University of New York. He has more than 100 publications and a best-selling book in chromatography. He is an editorial advisory board member of LCGC North America and the Chinese American Chromatography Association. Direct correspondence to: LCGCedit@ubm.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






