How Switching from Helium to Hydrogen Carrier Gas in Thermal Desorbers Enhances Gas Chromatography–Mass Spectrometry Systems
Analysis of polyaromatic hydrocarbons (PAHs) in air demonstrates that a thermal desorption–gas chromatography–mass spectrometry (TD–GC–MS) system can operate with hydrogen carrier gas as well as it does with helium.
Many semivolatile organic compounds (SVOCs) are considered hazardous to human health, including some phthalates (1) and benzo[a]pyrene (a five‑ring PAH), a toxic air pollutant of interest to regulators both sides of the Atlantic (2,3).
However, traditional reference methods for measuring SVOCs in ambient air (4) are cumbersome and prone to high uncertainty (5,6). They typically involve using large pumps to draw large volumes (up to hundreds of millilitres) of air through filters backed by polyurethane foam, XAD‑2, or other similar sorbents, then Soxhlet extraction and steam distillation followed by gas chromatography–mass spectrometry (GC–MS) analysis.
As thermal desorption (TD)–GC–MS technology has evolved over the years, its compatibility with increasingly higher‑boiling compounds has improved (7). Now, TD-based methods provide a reproducible, readily automated, and convenient alternative approach for monitoring SVOCs in ambient air in non‑dusty environments (8–16). Sample volumes in the order of 500 L are typically collected over a 24-h period using conventional small (personal monitoring) pumps at a rate of 350 mL/min. Subsequent TD–GC–MS analysis harnesses the SVOC recovery of modern TD technology and is suitable for GC‑compatible SVOCs with volatilities above n-C44 (b.p. > 500 °C). Example compound classes include polychlorinated biphenyls (PCBs), phthalates (up to and including didecylphthalate), polyaromatic hydrocarbons (PAHs), high-boiling hydrocarbons, and some polybrominated flame retardants (8–16).
With the latest enhancement to TD technology being certification for operation with hydrogen carrier gas, an investigation was carried out to see if there were any benefits or drawbacks of using hydrogen when measuring SVOCs. PAHs were selected as test compounds as they are particularly “sticky” and challenging. A PAH study using conventional helium carrier gas provided the basis for comparison (17).
Why Consider Switching to Hydrogen Carrier Gas?
Helium is a finite resource that is increasingly expensive and difficult to source as a GC carrier gas. In addition, it must be extracted and stored before being shipped around the world, giving it a high carbon footprint. Hydrogen, on the other hand, is simple to generate using water and electricity and would seem to be an obvious environmentally friendly alternative, securing against helium shortages in the long term and offering immediate cost savings. It also promises shorter analytical cycle times and faster sample throughput. It is important to test the impact of hydrogen carrier gas on system performance with SVOCs. No TD features or functions are compromised by using hydrogen and all multi-gas thermal desorbers can also be used with helium or nitrogen carrier gas without changing system hardware. Also, they can be connected to any hydrogen-ready GC and MS instruments.
Experimental
Using the chromatographic conditions reported in reference 17 as a starting point, clean sorbent tubes (Markes International “High-boiler” tubes: part number C2-CAXX-5138) were loaded with a 16-component PAH standard (10 ng/µL) using a calibration solution loading rig (Markes International; see reference 18 for method). The TD method was developed and optimized in a short iterative process (see reference 19 for method). The GC conditions selected for use with helium were translated to conditions for hydrogen carrier gas using software that is available free-of-charge online (20). The objective of this phase of the study was to achieve analyte recovery levels and chromatographic resolution with hydrogen carrier gas equivalent to those achieved with helium previously (17). The results are shown in Figure 1.
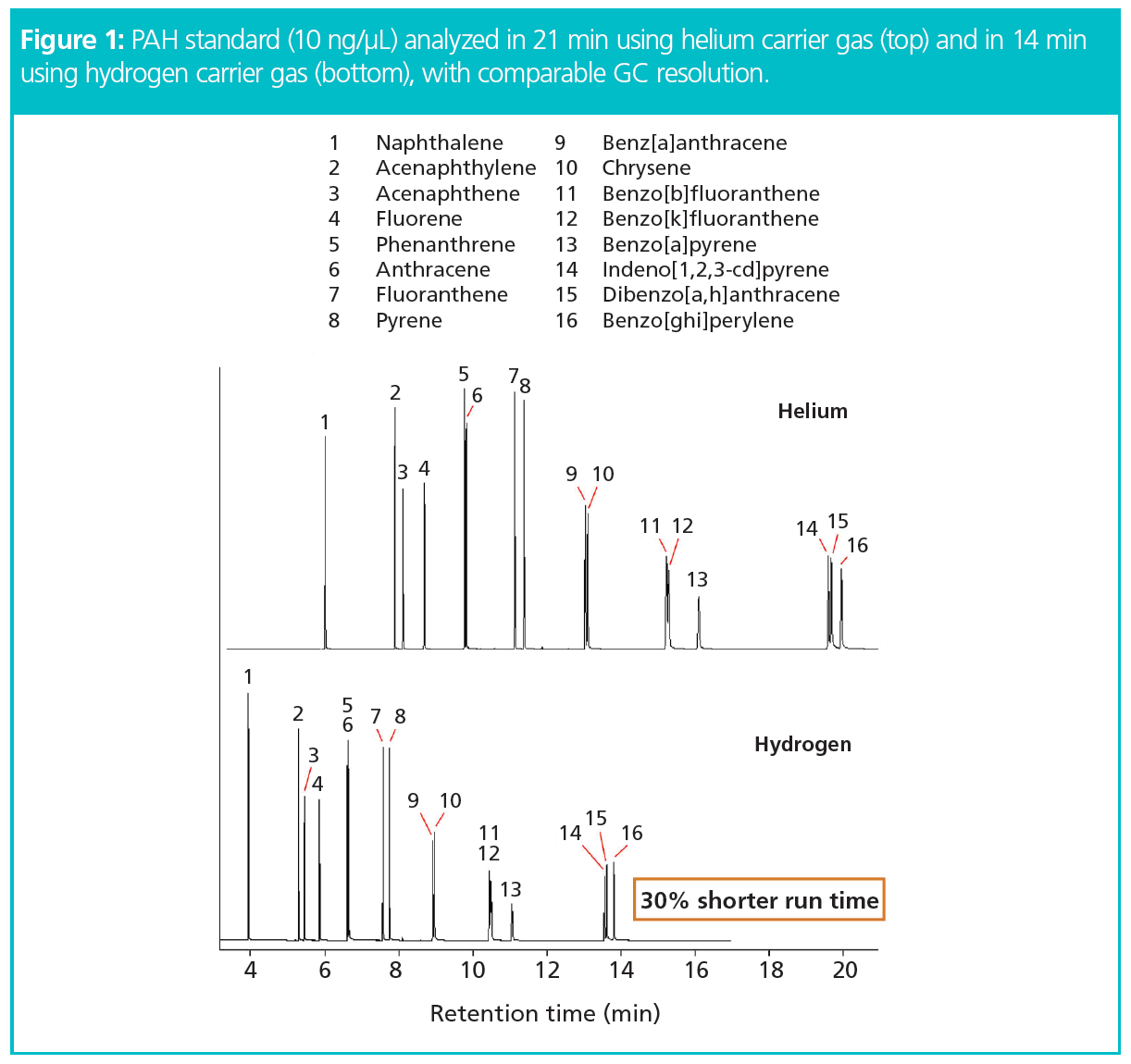
TD–GC–MS Conditions Using Helium: TD: instrument: TD100-xr (Markes International), focusing trap: ‘high-boiler’ tubes, tube desorption: 320 °C (15 min) with 50 mL/min tube and trap flow (no inlet split), trap desorption: –10 °C to 350 °C (10 min) at maximum heating rate, outlet split flow: 50 mL/min, flow path: 250 °C; GC: column: 30 m × 0.25 mm, 0.25-μm, DB-5ms (Agilent), column flow: 3 mL/min, oven ramp: 50 °C (1 min), then 15 °C/min to 100 °C, then 20 °C/min to 240 °C, then 10 °C/min to 260 °C (6 min), then 50 °C/min to 310 °C (5 min), inlet: 320 °C; MS: aux heater: 320 °C, ion source: 250 °C, SIM/scan analysis: m/z range 35–300.
TD–GC–MS Conditions Using Hydrogen: TD: instrument: TD100-xr Multi-Gas (Markes International), focusing trap: ‘high-boiler’ tubes, tube desorption: 320 °C (10 min) with 50 mL/min tube and trap flow (no inlet split), trap desorption: –10 °C to 350 °C (5 min) at maximum heating rate, outlet split flow: 50 mL/min, flow path: 250 °C; GC: column: 30 m × 0.25 mm, 0.25 μm, DB-5ms (Agilent), column flow: 3 mL/min, oven ramp: 50 °C (0.65 min), then 22.4 °C/min to 100 °C, then 30 °C/min to 240 °C, then 15 °C/min to 260 °C (6 min), then 74.5 °C/min to 310 °C (3.35 min), inlet: 320 °C; MS: aux heater: 320 °C, ion source: 250 °C, SIM/scan analysis: m/z range 35–300.
Results and Discussion
Analysis Time: The data obtained showed the expected significant gain in chromatographic efficiency without compromising resolution or sensitivity. Furthermore, it was found that complete recovery of the highest-boiling six-ring PAHs through the desorber could be achieved with 40% shorter desorption times using hydrogen compared with helium carrier gas—15 min with hydrogen versus 25 min using helium (Figure 2).
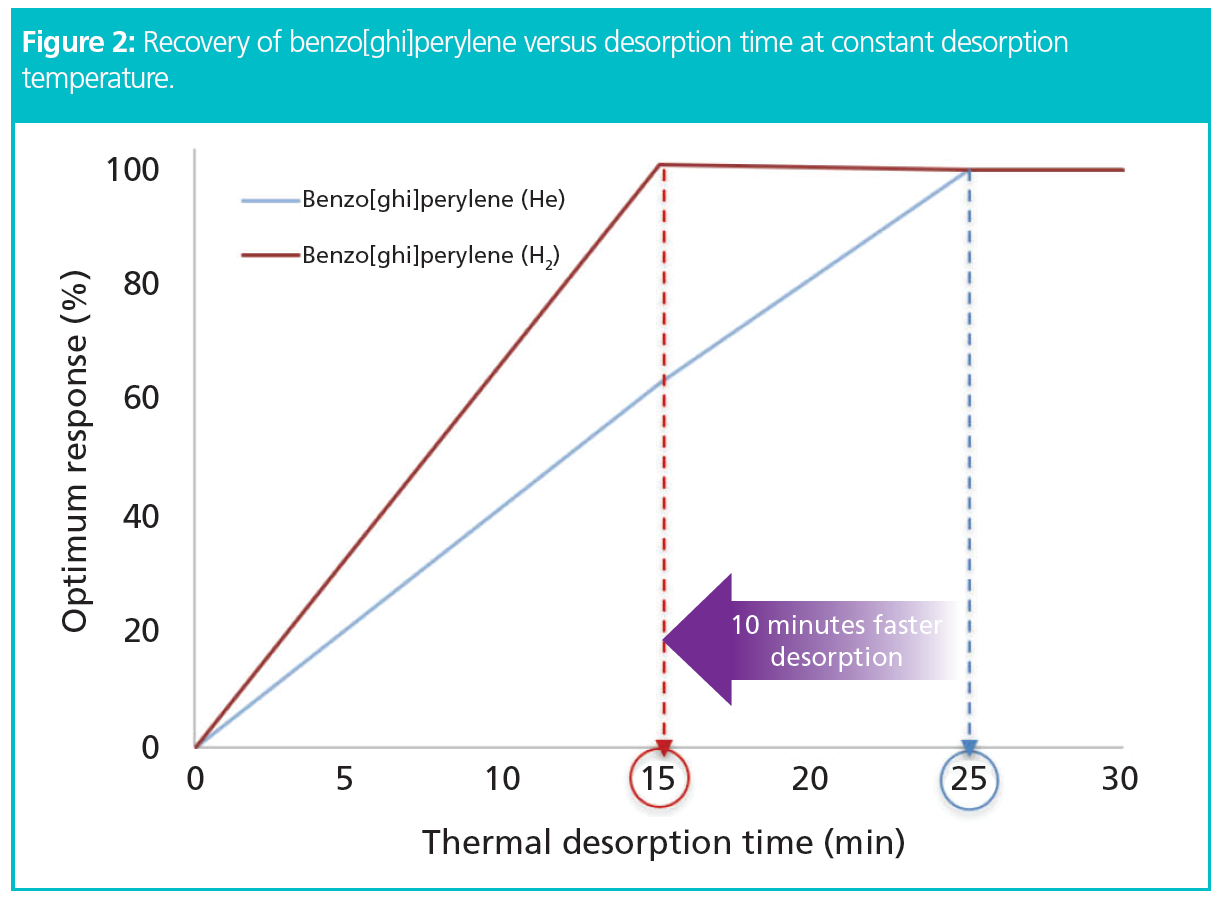
As the automated thermal desorber offered overlap mode—that is, it allowed the desorption of a subsequent sample to begin while GC–MS analysis of the previous sample continued—GC cycle times became the most important factor dictating system productivity. Figure 3 illustrates the improvement in sample throughput that can be achieved by using hydrogen carrier gas for this SVOC application. Broadly speaking, deployment of hydrogen carrier gas instead of helium allowed the analysis of at least one extra SVOC sample per hour.
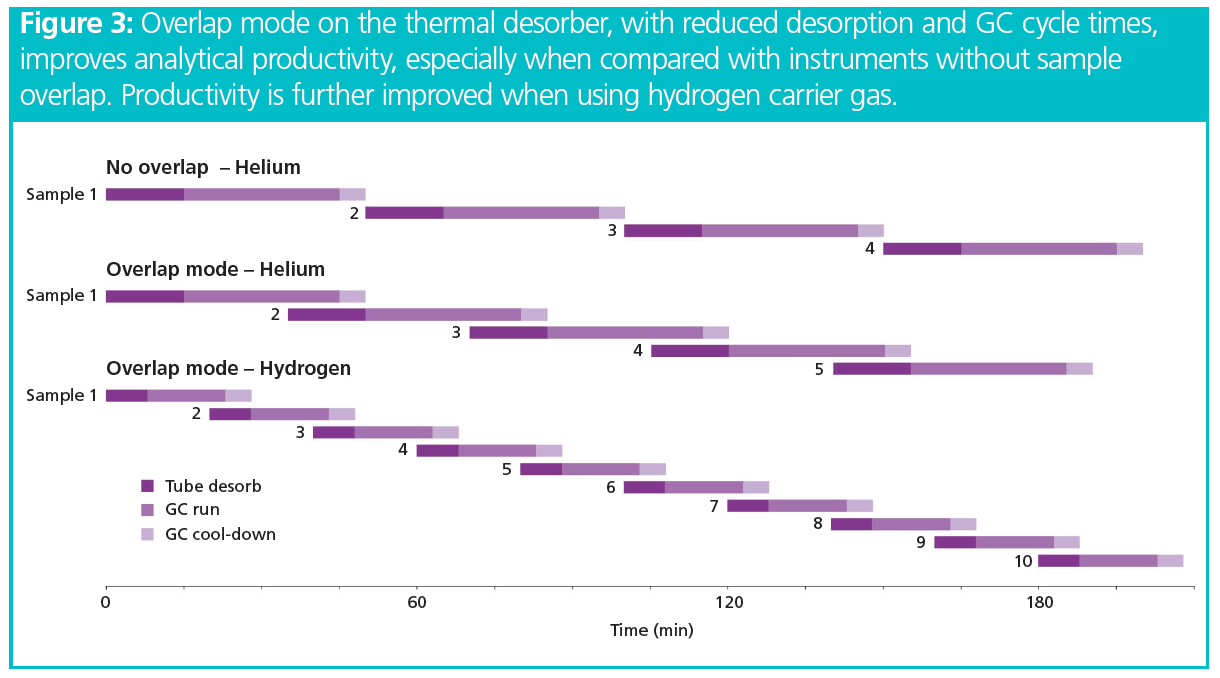
Assessment of Method Performance: System performance was evaluated
for peak shape at low levels, linearity, limits of detection and quantification
(LOD and LOQ), and reproducibility. The use of quantitative sample re-collection and repeat analysis was also evaluated as a means of validating analyte recovery.
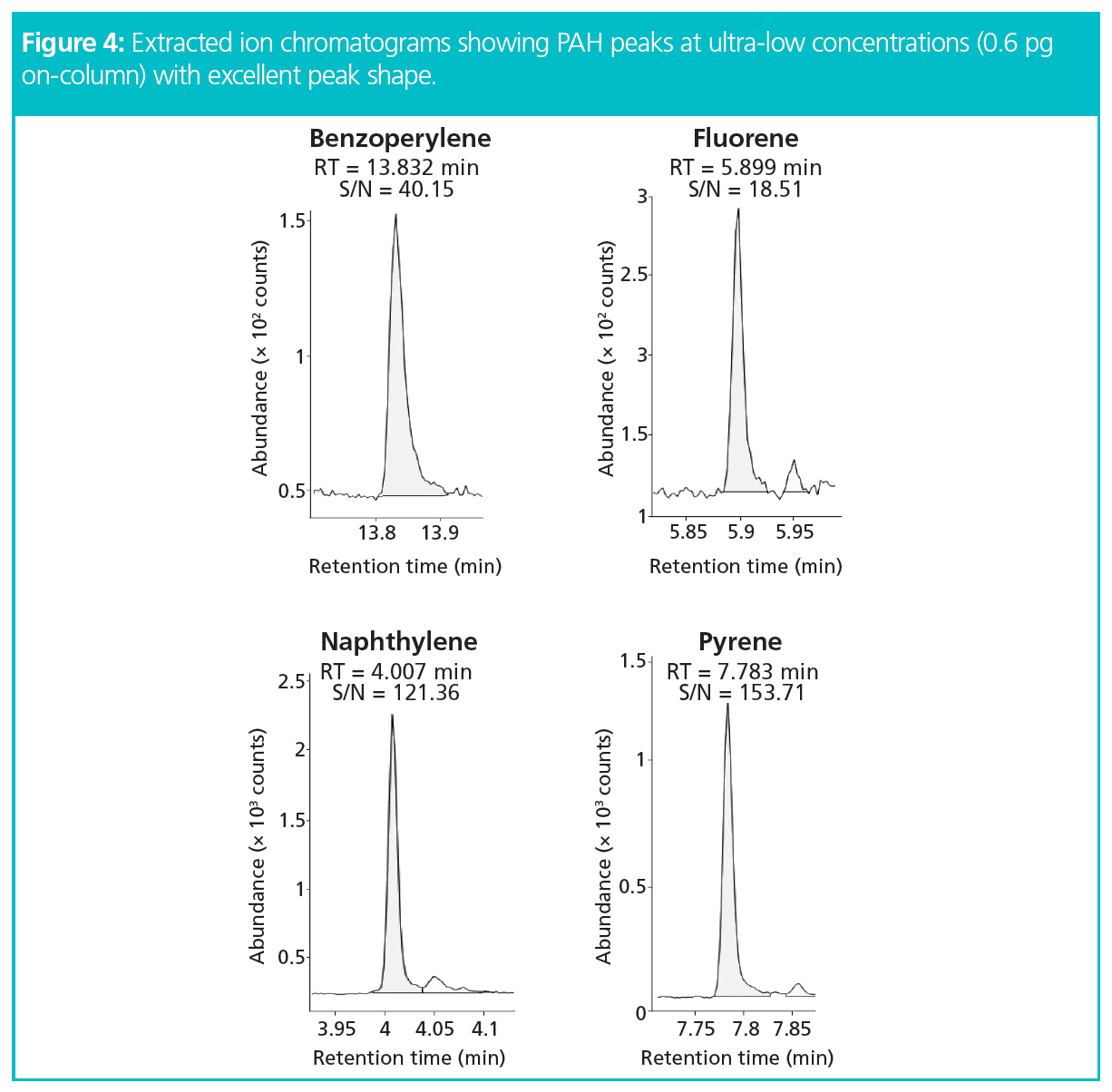
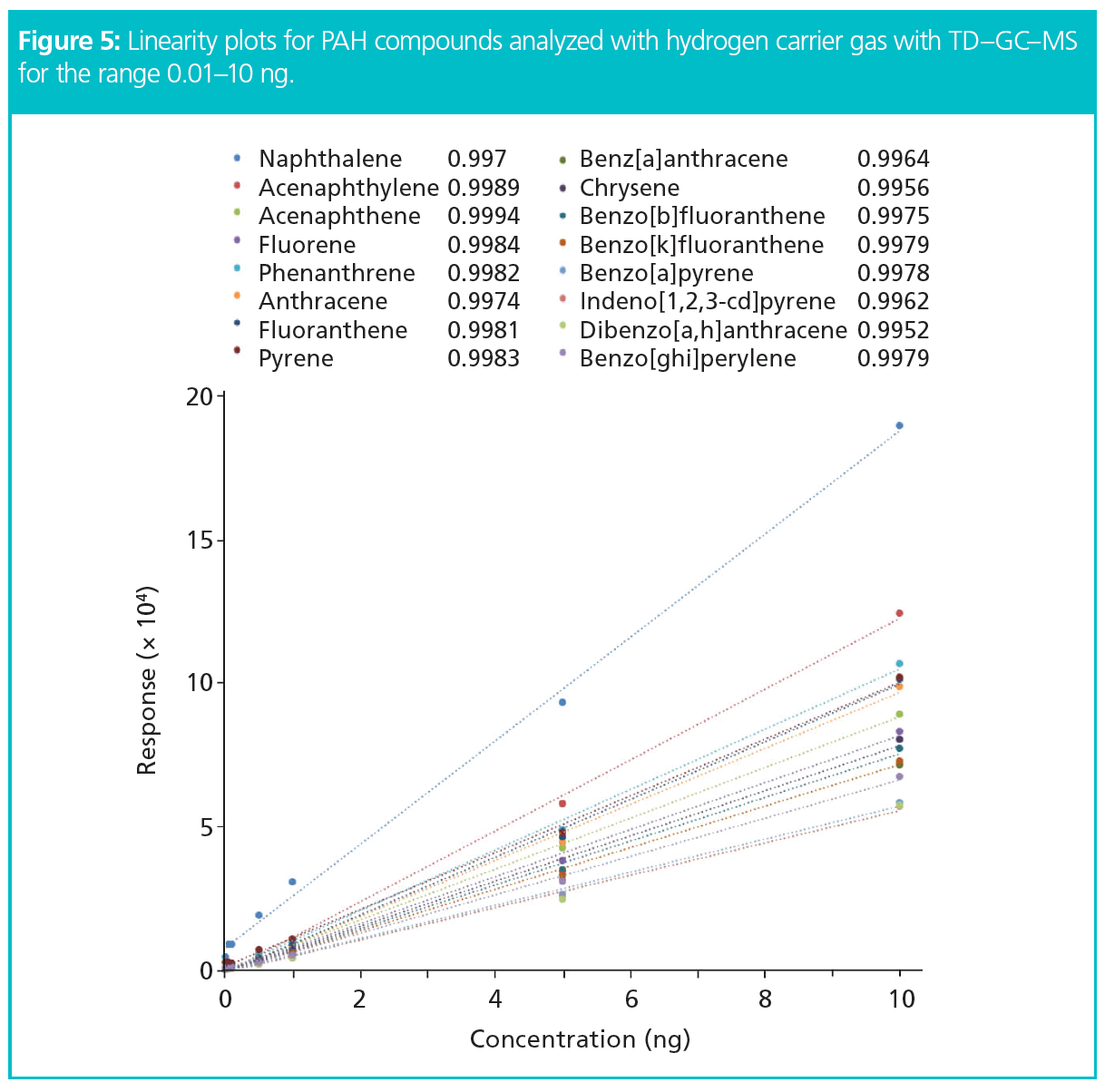
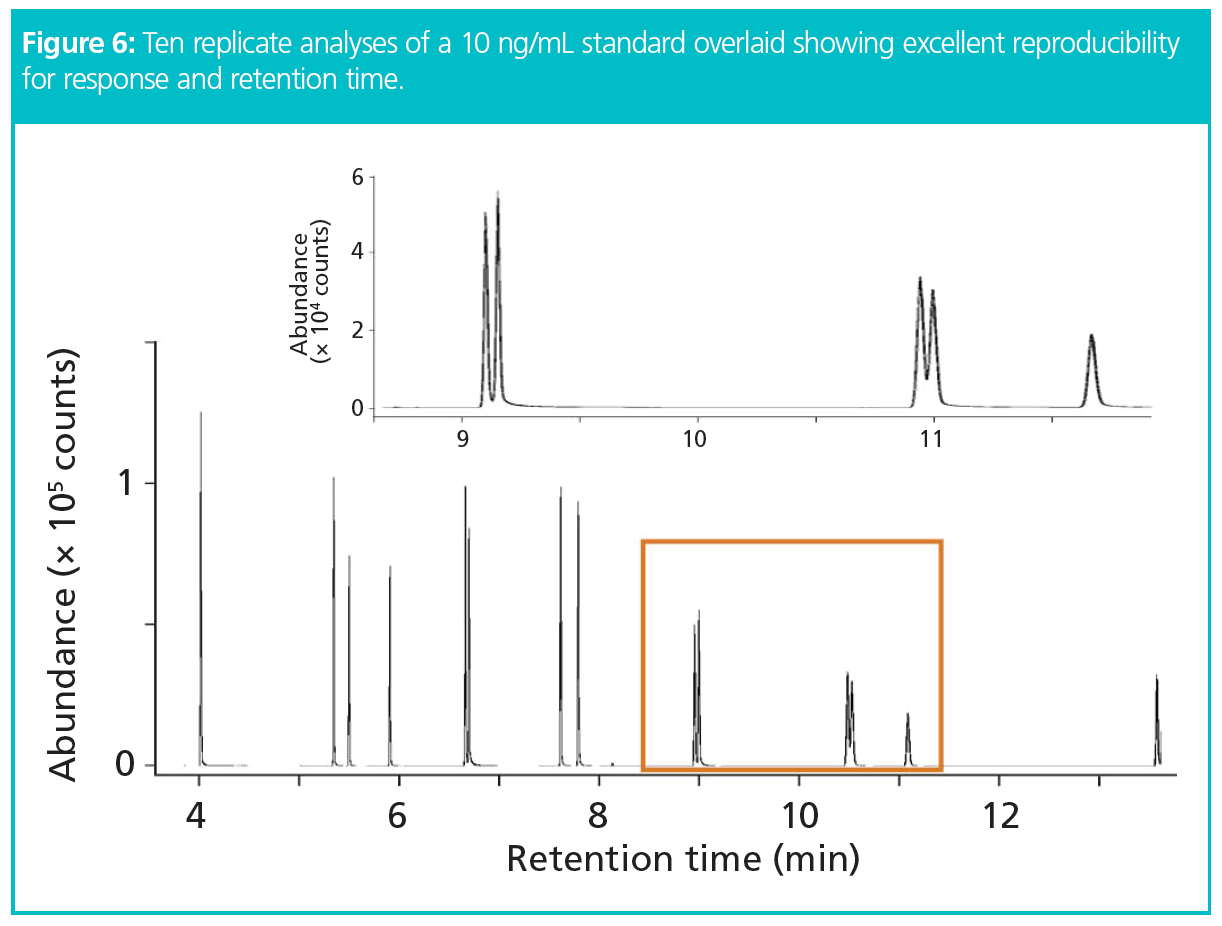
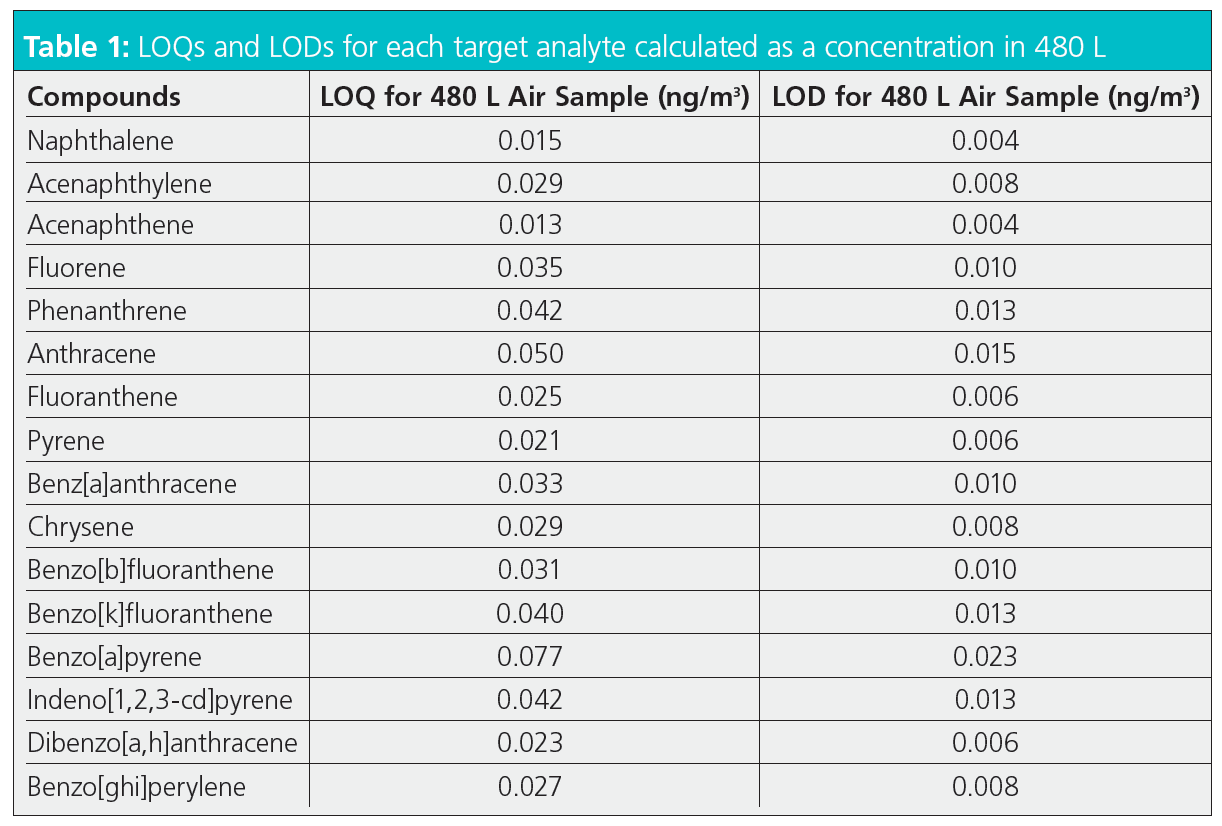
The results showed exceptional peak shapes, even at low picogram (pg) levels for each compound of interest. Figure 4 presents four examples of PAHs across the chromatogram from naphthalene to benzoperylene, showing excellent peak shapes even at 0.6 pg on the column. Linearity (R2) was greater than 0.995 for all compounds across the full volatility range for a concentration range of 0.01–10 ng standards (Figure 5). The results also showed exceptional sensitivity. Assuming air was sampled at 333 mL/min, allowing the collection of 480 L over 24 h (17), all LOQs were below 0.08 ng/m3 and all LODs were below 0.025 ng/m3 (Table 1). This demonstrates comparable results to those previously found with helium (17), and therefore no loss in sensitivity. Excellent reproducibility was obtained, as demonstrated in Figure 6 where 10 replicate analyses are overlaid and show a high degree of consistency for both retention time and intensity.
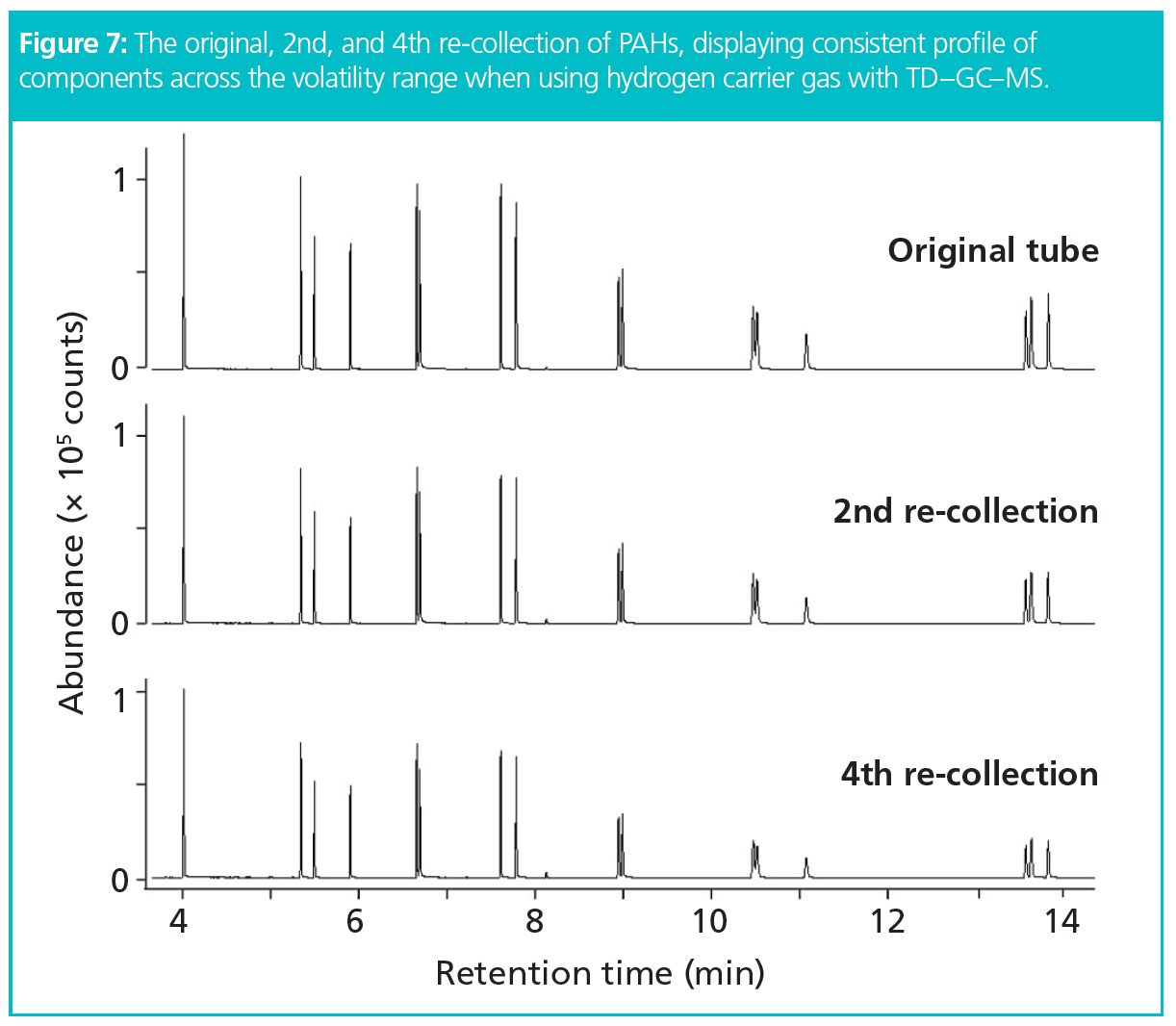
To validate analyte recovery, a standard (10 ng/µL) was re-collected and the analysis repeated several times. This process tests the entire TD–GC–MS workflow and is recommended in international standard methods such as ISO 16000-6 (21). If compound losses do occur, for example due to compound decomposition, hydrogenation, adsorption, or condensation, it would result in the affected peak area being smaller than expected in the repeat analysis, that is, smaller than that predicted from the split ratio or relative to other peaks in the mix. The data is shown in Figure 7 and demonstrates excellent recovery (17), that is, > 99% recovery across the PAH volatility range when using hydrogen as a carrier gas.
Conclusion
This study has shown that the excellent performance of multi-gas-enabled thermal desorption for PAHs can be comfortably replicated using hydrogen carrier gas. Further, the results from this study have shown that using hydrogen significantly speeds up the overall analysis, offering both improved desorption efficiency and faster chromatographic separations with no significant downsides in terms of loss in sensitivity, reactivity, or method reproducibility. Moreover, important analytical quality control checks, such
as quantitative sample re-collection for repeat analysis and validation of analyte recovery, have been shown to work just as effectively on the TD systems configured with hydrogen carrier gas as with helium.
In conclusion, it is important to consider that many other important advantages can be realized by switching to hydrogen. These include significant cost savings—hydrogen cylinders are between 6 and 10 times less expensive than helium cylinders and can be replaced by hydrogen generators for even bigger long-term savings. Lowering high-temperature desorption times on the TD and time spent by the GC column at maximum temperature protects system components, extending maintenance intervals and lowering levels of system background artefacts. The extra desorption efficiency of hydrogen can either be used to speed things up (as described in this study) or it can be used to achieve the same recovery within the same time but at lower temperatures. This is often preferred in trace-level studies, for example, where the most important factor is minimizing background rather than productivity or cycle times.
References
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH), https://www.legislation.gov.uk/eur/2006/1907/contents.
- Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel, and polycyclic aromatic hydrocarbons in ambient air, https://www.legislation.gov.uk/eudr/2004/107/contents.
- J. Chunrong, F. Xianqiang, L. Smith, and R. Rogers, Characterizing community exposure to atmospheric polycyclic aromatic hydrocarbons (PAHs) in the Memphis tri-state area, https://www.epa.gov/sites/default/files/2020-01/documents/memphis_pahs_study_final_report_08.pdf.
- EN ISO 16000-13, 2008, Indoor Air – Part 13: Determination of total (gas and particle-phase) polychlorinated dioxin-like biphenyls (PCBs) and polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDDs/PCDFs) – Collection on sorbent-backed filters, https://www.iso.org/obp/ui/#iso:std:iso:16000:-13:ed-1:v1:en.
- T.F. Bidleman, W.N. Billings, and W.T. Foreman, Environmental Science and Technology 20, 1038–1043 (1986).
- C. Schauer, R. Niessner, and U. Pöschl, Environmental Science and Technology 37, 2861–2868 (2003).
- E. Woolfenden, in Gas Chromatography, C.F. Poole, Ed. (Elsevier, 2021), pp. 267–323.
- E. Wauters, P. Van Caeter, G. Desmet, F. David, C. Devos, and P. Sandra, Journal of Chromatography A 1190, 286–293 (2008).
- B. Lazarov et al., Atmospheric Environment 79, 780–786 (2013).
- B. Lazarov et al., Environmental Science and Pollution Research 22, 18221–18229 (2015).
- Markes Application Note 053, The performance of thermal desorption for the analysis of high-boiling SVOCs.
- Markes Application Note 136, The performance of thermal desorption for the quantitative analysis of long-chain hydrocarbons.
- Markes Application Note 137, Quantitative determination of semi-volatile polychlorinated biphenyls (PCBs) using TD–GC–MS.
- Markes Application Note 138, A robust TD–GC–MS method for the determination of trace-level phthalate esters in air – Method validation and field study.
- Markes Application Note 139, High-performance analysis of polycyclic aromatic hydrocarbons (PAHs) by TD–GC–MS: Method validation and case-study.
- Markes Application Note 140, Quantitative analysis of airborne semi-volatile flame retardants using sorbent tube sampling with TD–GC–MS.
- Markes Application Note 139, High-performance analysis of polycyclic aromatic hydrocarbons (PAHs) by TD–GC–MS: Method validation and case-study.
- Markes Application Note 007, Preparing and introducing standards using thermal desorption.
- Markes Application Note 021, Developing and optimising tube-based thermal desorption methods.
- EZGC tool, Restek: https://ez.restek.com/ezgc-mtfc
- ISO 16000-6:2011 Indoor air — Part 6, Determination of volatile organic compounds in indoor and test chamber air by active sampling on Tenax TA sorbent, thermal desorption and gas chromatography using MS or MS-FID, https://www.iso.org/standard/52213.html.
Laura Miles is a senior application specialist in the thermal desorption business unit at Markes International. Laura is responsible for developing new methods and testing Markes’ suite of thermal desorbers for new and emerging applications. Laura joined Markes as a customer support specialist in 2014 before moving to work in application development. As part of her current role, Laura works closely with key opinion leaders in collaborations across a variety of market areas, and she has a particular specialism in environmental analysis, breathomics, and defence and forensics.
Elinor Hughes obtained her B.Sc. in chemistry and Ph.D. in organic chemistry at Bangor University, UK. After working for a chemical manufacturing company for three years, she moved to the Royal Society of Chemistry where she worked in journals publishing for six years and on Chemistry World magazine for four years. This was followed by five years as a freelance copyeditor and science writer. Her current role is technical copywriter at Markes International.
E-mail: enquiries@markes.com
Website: www.markes.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.
The Role of SPME Combined with GC–MS for PFAS Analysis
Published: March 25th 2025 | Updated: March 25th 2025Emanuela Gionfriddo and Madison Williams from University at Buffalo, the State University of New York, NY, USA discuss the important role that solid-phase microextraction (SPME) techniques with gas chromatography mass spectrometry (GC–MS) can play in the analysis of per- and polyfluoroalkyl substances (PFAS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









