How Fast Can a Gradient Be Run?
LCGC North America
An exploration of how different variables affect a separation
For method development or routine analysis, the minimum gradient time may be a limit of throughput.
When you are developing a new liquid chromatography (LC) method, speed often is of primary interest — both speed in development and speed in terms of run time for the final method. With the expanding interest in quality by design (1), we would like to know how different variables affect a separation — both to ensure that a method is robust and to provide information for method adjustment when necessary. Gradient elution often is a first choice for method development experiments because the run times are defined and gradient data can be converted to isocratic conditions with the help of software programs such as DryLab (Molnar Institute, Berlin, Germany).
Because speed is of interest, it is good to know just how fast we can make gradient runs and still have "good" chromatography. After all, we don't want to just blast the sample through the column with no chance of separation. Fortunately, there is a well developed theoretical basis for gradient elution (2) that allows us to make predictions of conditions that will satisfy our goals.
Selecting a Gradient Time
Before we look at gradients, let's consider isocratic separations, in which the organic solvent (%B) in a reversed-phase mobile phase is at constant concentration. For isocratic conditions, an ideal separation will have retention factors, k, for all analytes in which 2 < k < 10, but it is sometimes hard to fit all peaks within this range, so we settle for 1 < k < 20 for acceptable chromatographic behavior. For k < 1, there is a risk of interferences from unretained material from the sample matrix and retention is very sensitive to small changes in %B. For k > 20, peaks become broad, and thus harder to detect, and the run time gets inconveniently long.
With gradient separations, the k-value changes as the sample travels through the column. At the beginning of the separation, the %B is low and k is large; as the peak moves along the column, the %B rises and k drops. Thus, k in the isocratic context is a moving target during a gradient run. For this reason, we use k* to represent a single number in gradients; this can be thought of as an average k-value. For gradients, k* is approximately the same for all peaks in the chromatogram and can be estimated as follows:
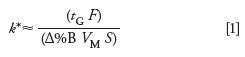
where tG is the gradient time (in minutes), F is the flow rate (mL/min), Δ%B is the gradient range (5–95% = 0.9), VM is the column volume (mL), and S is the slope of a plot of isocratic log k vs. %B. S can be estimated as follows:
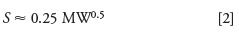
where MW is the molecular weight. A value of S = 5 will be used in the present discussion, representing a typical value for compounds of MW < 1000 Da. Because S-values are approximately (but not exactly) the same for a sample mixture, k*-values (equation 1) also will be approximately the same for all peaks in a run under the same gradient conditions. Like isocratic runs, it is desirable to have 2 < k* < 10 for gradients. If a pair of gradient runs is desired for use with predictive calculations, such as with DryLab, k*-values differing by 2–3-fold are recommended (for example, runs with k* = 3 and 6). For fast runs with "good" chromatography, the topic of this discussion, we'll use k* = 2.
We can rearrange equation 1 to estimate the gradient time required for various chromatographic conditions:
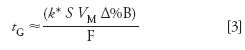
An Example
Let's look at an example using equation 3 for a separation using a 150 mm × 4.6 mm, 5-µm particle diameter (dp ), column run at 2 mL/min (F = 2) and a gradient range of 5–100%B (Δ%B = 0.95). An estimate of the column volume is:
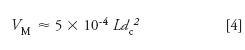
where L is the column length and dc is the column diameter, 150 mm and 4.6 mm here. (This assumes a total column porosity of ≈65%.) So VM ≈ 1.6 mL for the present example. For k* = 2, we can calculate:
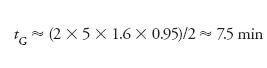
You can find this summarized in the first line of Table I. (Note that in this calculation and all those in Table I, I've rounded the numbers, so if you repeat these calculations, your results may vary slightly.)
It might also be interesting to estimate the pressure, P, under these conditions. We can do this (in psi) with
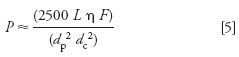
(equation 2.13a in reference 3) where η is the mobile phase viscosity in cP. For gradients, we need to use the highest viscosity during the run. For acetonitrile as the B-solvent and 30 °C, η = 0.9 (Table I.5 of reference 3). This gives a maximum pressure of ≈ 1275 psi; in my experience, this calculation is a bit low for real columns, so I've multiplied values from equation 5 by 1.5 for Table I to give a better feel of the pressure that is likely to be observed.
Some Other Column Configurations
I've made similar calculations as above and summarized these in Table I for several other popular column configurations. The columns all have approximately the same number of theoretical plates, N ≈ 10,000 (±10%) under realistic conditions, so they should give the same separations in all cases; the exception is the 50 mm × 2.1 mm, 3-µm column with N ≈ 5000. The flow rates were chosen to give pressures of ≈ 2000–3000 psi (135–205 bar) for conventional LC equipment and 10,000–15,000 psi (680–1020 bar) for ultrahigh-pressure liquid chromatography (UHPLC), which represent "typical" method conditions.
You can see in Table I that for conventional methods using 100–150 mm columns with 3- or 5-µm particles, gradient times of 7–8 min are used for k* = 2. For a second calibration run for retention modeling software, use a run threefold longer (20–25 min). For the 50 mm × 2.1 mm, 3-µm column popular for LC–mass spectrometry (MS), a gradient of ≈2 min is required.
Under UHPLC conditions with a 2-µm column (last two rows in Table I), run times of just under 2 min are required. For comparison, I've included a 2.5-µm shell-type column (third line from the bottom of Table I); these columns have pressures reflected by the particle size, but performance more like a 2-µm particle. In the configuration chosen, gradient times are about the same as UHPLC columns, but with a somewhat lower pressure.
So far so good. It looks like we can get "good" chromatography with runs of 7–8 min on conventional LC equipment and in 2 min for UHPLC conditions. These are fast enough to give us the ability to screen many possible variables in a reasonable amount of time. However, there are a couple additional factors that we'll consider next.
Gradient Delay
One factor to take into account with fast gradients is the gradient delay. This is determined by the dwell volume, VD , of the system, which comprises the mixer, connecting tubing, sample loop, and for low-pressure mixing systems, the pump head volume. The dwell volume should be measured for each LC instrument; directions for this were given in a previous "LC Troubleshooting" installment (4). Typical dwell volumes for conventional high-pressure mixing LC systems are in the 1.5–3.0 mL range; their low-pressure mixing counterparts generally are slightly larger, with 2.5–4.0 mL dwell volumes. High-pressure mixing systems that have been tuned up for LC–MS work and UHPLC systems typically have dwell volumes of 0.3–0.5 mL, whereas low-pressure mixing UHPLC systems have 1.0–1.5 mL.
The practical impact of the dwell volume on the run time is that it extends the run because the dwell time, tD — the time it takes for the gradient to reach the column after it is started — is added to the gradient time. This is shown in the third column from the right of Table I. I've assumed a 2.0-mL dwell volume for conventional LC systems (first three rows) and 0.4 mL for the LC–MS and UHPLC configurations. Note that for the 4.6-mm columns and 1.5–2 mL/min flow rates (first two lines of Table I), the added time is 15–20%, but if a 100 mm × 2.1 mm column is used, the total run time is nearly doubled by the gradient delay. With the 2.1-mm i.d. columns used for LC–MS or UHPLC, the delay can be substantial, 25–40% additional time for the run. For some instruments, it may be possible to delay the injection until the gradient reaches the column, but the dwell time must be paid for sometime — in such cases, it is moved from within the run to between consecutive runs. Additionally, the dwell volume must be thoroughly flushed out when changing the mobile phase from one condition to another during method development.
It is clear that it is desirable to minimize the dwell volume to maximize throughput with gradients. In some cases it may be possible to reduce the dwell volume by changing system plumbing, but there is a minimum dwell volume that is characteristic of each LC system.
Gradient Distortion
Another potential problem related to the dwell volume is gradient distortion. This is illustrated in Figure 1. In each case, the gradient program is shown with a dashed line and the solid line shows the actual gradient generated by the LC system. In the top case, the gradient is ramped at 3%/min and in the lower run, 14%/min. You can see that in one case, the actual gradient closely follows the intended one, whereas in the steep gradient, there is considerable distortion in the actual gradient. Clearly the lower case is undesirable. Such gradients are very instrument-dependent and will be hard to transfer. Based on these data (5), it was concluded that as long as the gradient volume is at least twice as large as the dwell volume, gradient distortion should be minimal. The upper plot has a gradient volume twice the dwell volume, whereas the ratio for the lower one is only 0.3.
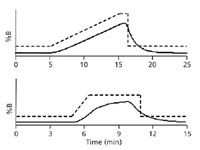
Figure 1: Gradient distortion comparing 3%/min (top) and 14%/min (bottom) gradients. Dashes: gradient program; solid line: actual gradient. Data are from reference 5.
The next-to-last column in Table I shows the gradient volume, VG (= tG × F), for each gradient condition. The maximum recommended dwell volume (half the gradient volume) is shown in the far right column of Table I. We can conclude that gradient distortion is not likely to be a big problem with conventional LC equipment used with 4.6-mm i.d. columns or with LC–MS or UHPLC equipment used with 2.1-mm i.d. columns. In either case, however, when smaller diameter columns are used, gradient distortion is a factor that should not be ignored. In any case, it is wise to check to be sure that your LC system will generate a distortion-free gradient under the fastest desired gradient conditions. This can be done in the same manner as the dwell volume measurement discussed above (4), but using the desired gradient conditions.

Table I: Characteristics of various gradient configurations
Conclusions
Fast gradients can be generated reliably with conventional, LC–MS, and UHPLC-type LC equipment if certain precautions are taken. Fast gradients allow for rapid screening and optimization of several variables during method development and the creation of short run times for routine analysis. It is important to know the dwell volume of each gradient system so that gradient delay can be taken into account. Also a check should be made that gradient distortion is not observed, or the transfer of such gradients may be problematic. It should be obvious that longer, larger-volume gradients will be less subject to distortion, so only the shortest gradients anticipated need to be checked for potential distortion problems.
John W. Dolan "LC Troubleshooting" Editor John Dolan has been writing "LC Troubleshooting" for LCGC for more than 25 years. One of the industry's most respected professionals, John is currently the Vice President of and a principal instructor for LC Resources, Walnut Creek, California. He is also a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to John.Dolan@LCResources.com.

John W. Dolan
References
(1) International Committee on Harmonization, ICH Q8 Pharmaceutical Development (May 2006).
(2) L.R. Snyder and J.W. Dolan, High-Performance Gradient Elution (Wiley Interscience, New York, New York, 2007).
(3) L.R. Snyder, J.J. Kirkland, and J.W. Dolan, Introduction to Modern Liquid Chromatography (Wiley, New York, New York, 3rd Edition, 2010).
(4) J.J. Gilroy and J.W. Dolan, LCGC 22(10), 982–988 (2004).
(5) G. Hendricks, J.P. Franke, and D.R.A. Uges, J. Chromatogr. A 1089, 193 (2005).

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










