A Highly Sensitive Method for the Analysis of Tamsulosin (Flomax) in Human Plasma
The Application Notebook
A highly sensitive analytical method for the analysis of tamsulosin in human plasma has been developed for use in bioanalytical studies. The solid-phase extraction (SPE) and UPLC–MS–MS methodologies are described, as well as performance against validation parameters.
Erin E. Chambers, Jessalynn Wheaton and Diane M. Diehl, Waters Corporation, Milford, Massachusetts, USA.
A highly sensitive analytical method for the analysis of tamsulosin in human plasma has been developed for use in bioanalytical studies. The solid-phase extraction (SPE) and UPLC–MS–MS methodologies are described, as well as performance against validation parameters.
Introduction
The challenges of developing a bioanalytical method revolve around meeting the rigorous criteria set forth in the FDA Guidance for Industry for Bioanalytical Method Validation. Methods need to be acceptable in terms of linearity, sensitivity, selectivity, accuracy and precision, stability and carryover. Recently, the FDA has included a more comprehensive set of guidelines relating specifically to the assessment and quantification of matrix effects as well. The result is that bioanalytical methods need to be more reliable and reproducible than ever, placing increased burden on method development scientists.
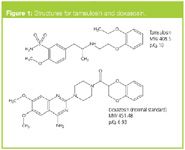
Figure 1
Tamsulosin, the active ingredient in Flomax, is a highly selective alpha1-adrenoceptor antagonist used to treat enlarged prostate in men. We have developed an analytical method that achieves an LLOQ of 10 pg/mL for tamsulosin in human plasma. This LLOQ is 10× lower than previously published.1
A combination of mixed-mode SPE and chromatography performed with a UPLC system were employed to increase sensitivity and to more selectively separate the analytes from matrix components. In addition to selectivity, the use of the Oasis µElution format significantly increased the speed of sample processing. An entire 96-well plate could be processed in under 30 minutes, or under 20 seconds per sample.
Structures for the analyte and its internal standard, doxazosin, are shown in Figure 1.
Experimental
System: Waters ACQUITY UPLC System with a Waters Quattro Premier XE triple quadrupole mass spectrometer operated in positive ion MRM mode
Column: ACQUITY UPLC BEH C18, 2.1 × 50 mm, 1.7 µm
Mobile phases: A: 0.1% HCOOH in water B: MeOH
Gradient: 95% A to 25% A in 1 min, hold 0.5 min, ramp to 2% A in 0.1 min and hold for 0.4 min return to initial (2.5 min total cycle time)
Flow-rate: 0.6 mL/min
Injection: 40 µL
Temperature: 35 °C
Desolvation gas flow: 800 L/hr
Source temperature: 120 °C
Desolvation temperature: 450 °C
Collision cell pressure: 2.6 × 10–3 mbar
MRM transitions: tamsulosin 409 228; doxazosin 452 344
Solid-Phase Extraction (SPE)
SPE device: Oasis MAX (Mixed-mode Anion eXchanger) µElution 96-well plate
Condition: 200 µL MeOH
Equilibrate: 200 µL water
Load: 300 µL human plasma diluted and acidified with 300 µL 4% H3PO4 in water
Wash 1: 200 µL 10 mM ammonium acetate, pH 5
Elute: 2 × 25 µL ACN
Dilute: 50 µL H2O Directly inject 40 µL
Results and Discussion
Mixed-mode SPE was chosen because it is a highly selective sample prep technique and has been shown to be a very powerful tool in reducing matrix effects for bioanalytical assays.2 Minimization of variability across different sources of matrix (i.e., individual subjects in clinical studies), has been a major point of discussion in relation to incurred sample reanalysis (ISR). Many researchers believe matrix effects arising from sample variability are one of the primary causes for failure in repeat analyses. Variability of matrix effects across multiple lots of human plasma in this assay was less than 15%.
SPE recovery and RSDs were calculated for both tamsulosin and the internal standard, doxasosin (Figure 2). Though both compounds are basic in nature, they have very different pKa's. The pKa's for tamsulosin and doxazosin are approximately 10 and 7, respectively. To obtain high recovery for both analytes in a single elution, while still taking advantage of the selectivity of a mixed-mode SPE sorbent, the analytes must both be neutral to bind solely by a reversed-phase mechanism. This was accomplished by washing with a pH 5 buffer prior to elution. Additional interferences bound by ion-exchange remained on the sorbent. Sample processing was performed in under 20 seconds per sample using the 96-well µElution format. Analytes were pre-concentrated using the plate to achieve the desired detection limit without the need for an evaporation and reconstitution step.

Figure 2
The method is linear (1/x weighting) for the determination of tamsulosin in human plasma from 0.01 to 10 ng/mL. Correlation coefficients were greater than 0.998 and % deviations from the actual concentrations were less than 10%, including at the lower limit of quantification (LLOQ). Sensitivity at the LLOQ of 0.01 ng/mL is shown in Figure 3. Calculated concentrations for QC's at 0.015, 0.25 and 7.5 ng/mL were all within 12% of expected values.

Figure 3
Conclusions
A rapid, sensitive and reliable UPLC–MS–MS method was developed for quantification of tamsulosin in human plasma. The combination of mixed-mode SPE processing in a 96-well, µElution format with 2.5 minute UPLC cycle times yielded a sample throughput of over 400 samples a day without compromising data quality.
References
1. N.V. Ramakrishna et al., Biomed Chromatogr., 19(10), 709–719 (2005).
2. E. Chambers et al., J. Chromatogr. B, 852(1–2), 22–34 (2007).
©2008 Waters Corporation. Waters, The Science of What's Possible, Oasis, UPLC, ACQUITY UPLC and Quattro Premier are registered trademarks of Waters Corporation.
Flomax is a registered trademark of Boehringer Ingelheim Pharmaceuticals Inc.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 478 1990
Website: www.waters.com

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.










