Highlights of HPLC 2017 Jeju
LCGC North America
A summary of the findings presented at the HPLC 2017 Jeju conference, reviewing how they fit into overall liquid-phase separation trends and the outlook for the future of these technologies
The HPLC 2017 Jeju conference that was held in November 2017 included valuable talks on liquid chromatography column technology and microfluidics. Here, we summarize the findings presented, reviewing how they fit into overall liquid-phase separation trends and the outlook for the future of these technologies.
Once every two years, the popular International Symposium on High Performance Liquid Phase Separations and Related Techniques (better known as HPLC) series moves its fall meeting to Australasia. The 46th HPLC Asian version was held November 5–9 in Jeju, South Korea. Jeju is a beautiful resort island off of the southern coast of South Korea. Chaired by Professor Doo Soo Chung of the Chemistry Department of Seoul National University, HPLC 2017 Jeju assembled more than 900 delegates from 35 countries, with a high percentage from Souh Korea, China, and Japan. It was held in the modern and large Jeju International Convention Center (Figure 1), where two other scientific meetings were held at the same time: the Asia Pacific International Chapters Conference (APICC) and the smaller International Union of Pure and Applied Chemistry (IUPAC) Symposium on Water and Environmental Analysis meeting. The APICC was sponsored by the American Chemical Society, which has chapters in 10 Australasian countries with 12,000 members. ACS President Allison Campbell was on hand to address the joint crowd.

Figure 1: Jeju Island with the HPLC 2017 Jeju International Convention Center venue in background.
Trends in Liquid-Phase Technology and Techniques
Obviously, high performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC) were the predominant technologies in the Symposium, but sample preparation, the use of electrodriven techniques, microfluidics, multidimensional techniques and, of course, mass spectrometry (MS) interfacing were strongly evident. From a perusal of the oral presentations, as in previous symposia, column technology was, by far, the strongest area of technology development.
An emphasis on monoliths, superficially porous packings, hydrophilic interaction chromatography (HILIC), multimodal chromatographic materials, and the potential of three-dimensional (3D) printing of complete columns was noted. Because of space limitations, I report only on the areas of column technology and microfluidics that caught my attention as significant advances.
Monoliths
Monoliths still command a lot of development work. In recent years, polymeric monolith experts have been trying to come up with polymeric columns that work well for both large and small molecules while silica monolith experts have been trying to extend the application range of silica monoliths to fit larger biomolecules. In one of the plenary lectures, Frank Svec of the Lawrence Berkeley National Laboratory, a longtime proponent of polymeric monolithic columns, presented the results of unbiased tests of the two main types of commercial monolithic columns. Using a series of small-molecule and large-molecule test probes on both reversed-phase silica-based (Chromolith, Merck) and polymer-based (ProSwift, Thermo Fisher) monolithic columns, he and his coworkers were able to find the optimum column type to match the analyte size range. The test probes were small organic molecules, mid-sized peptides, standard proteins, and a mixture of IgG with a small profen drug. After dispelling some of the myths (lack of reproducibility, especially for capillary columns and lack of robustness, overloading of polymeric columns) of monolith columns with actual data, he went on to examine these commercial columns. Basically, although there are a few exceptions, the bottom line of the study is that the silica monoliths perform best for small molecules and small-to-mid size peptides while the polymeric monoliths are best for large molecules such as proteins. Why the difference? It most likely involves the morphological differences, where the polymeric packings lack mesopore structure, which leads to lower surface areas, and the silica-based monoliths have mesopores, which provide a higher surface area and thus lower recovery for larger molecules. Also, the commercial polymeric monolithic packings, while compared in this study in the reversed-phase mode, are available for more separation modes, including ion exchange, hydrophobic interaction, hydrophilic interaction, and bioaffinity. Both types can result in fast separations.
Despite the studies of Svec, commercial studies of wide-pore silica monoliths for large biomolecule separations is continuing. A study by Merck chemists investigated various chemical functionalities (C4, C8, C18, epoxy, and protein A) bonded onto a 300-Å monolith with 2-µm macropores. The 300-Å mesopores are recommended for larger molecules to prevent analyte exclusion while the macropores provide excellent permeability, which allows for higher flow rates. For example, at 60 °C with a 100 mm × 4.6 mm column using an aqueous trifluoroacetic acid–acetonitrile mobile phase, at 4 mL/min, a pressure drop of only 32 bar was observed. The epoxy phase (3.2-µmol/m2 coverage, 200-bar maximum pressure) can be used to immobilize various ligands and the protein A (binding capacity: 35–45 mg/mL) has a high affinity for monoclonal antibodies. As far as immobilized groups, they have also been able to incorporate chiral, HILIC, and protein G functionality, among others. They showed some nice results on monoclonal antibodies. Another silica-monolith study involving biomolecules was presented by L. Zhang and coworkers from the Dalian Institute of Chemical Physics, China. They synthesized a periodic mesoporous hybrid monolith containing vinyl groups that are distributed throughout the skeleton. This monolith could be easily functionalized by click chemistry reactions. This monolithic column was successfully used for separating the intact proteins of HeLa cells. Based on the above results, perhaps there is still an opportunity for silica monoliths in large-molecule biochromatography.
Other papers involving monoliths included an interesting silica-based monolith for which Takuya and coworkers from Kyoto University, Japan, developed a normal-phase novel separation medium based on immobilized C70-fullerene. The capillary column showed specific retention for polycyclic aromatic hydrocarbons because of strong dispersion forces involving p-p stacking. In addition, a hemispherical molecule, corannulene, was significantly retained on this C70 column attributed by the authors to be a deflection of the p electron density in C70. Professor Koji Otsuka, also of Kyoto University, developed a novel separation medium, a spongy monolith, based on poly(ethylene-co-glycidyl methacrylate). It was prepared by blending a thermoplastic resin above its melting point with water-soluble pore templates. After removing the pore template with a water-wash, the resultant monolith contained flow-through pores greater than 10 µm in diameter. A column made of this material separates based on hydrophobic or ion-exchange interactions. The monolith could be derivatized for further selective interactions.
Multimodal Chromatography and Mixed-Mode Chromatography
Multimodal stationary phases have become a popular way of achieving selective interactions on a single packing material. An inadvertent example of multimodal chromatography is the use of a C18 phase at an intermediate pH where silanols can be ionized providing a negatively charged surface and undergo ionic interactions with an analyte's cationic functionality as well as hydrophobic interaction with the C18 group. However, other intentional multifunctional groups on the surface can lead to more desirable multiple selective interactions. X. Liu of Thermo Fisher Scientific gave a nice overview of mixed-mode chromatography (MMC) and showed examples of bimodal and trimodal surface chemistries to solve a variety of application problems. A unique trimodal phase consisting of an inner-pore area modified with an organic layer that provides both reversed-phase and anion-exchange properties and an outer-pore surface modified with cation-exchange functionality was used to separate various pharmaceutical compounds. Further enhancements in selectivity can be achieved by adjusting mobile phase buffer concentration, pH, and solvent content-either jointly or independently.
Kelly Zhang of Genentech gave a practical talk on the use of MMC in pharmaceutical analysis where controlled and purposeful multimode interactions are used to separate difficult compounds. MMC is complementary to reversed-phase chromatography and can replace ion-exchange, normal-phase, and hydrophilic interaction chromatography in many applications and can separate small polar charged compounds not typically retained by reversed-phase chromatography. Using case studies performed in her laboratory, she used both bimodal and trimodal commercial columns to analyze cations along with the active pharmaceutical ingredient (API), raw material identification and verification, residual metal analysis, PEG-maleimide conjugated intermediates, and polysorbate molecular heterogeneity in monoclonal antibody formulations. Professor Atilla Felinger of the University of Pecs, Hungary, used zwitterionic chiral stationary phases instead of weak anion and strong cation exchangers for the separation of small, charged chiral molecules. The bimodal interactions between mefloquine enantiomers, a malaria drug, and these phases showed increased selectivity enhanced by the use of a protic solvent.
Multidimensional LC
In general, two-dimensional (2D)-LC is becoming more mainstream since major LC instrument companies now have easy-to-use accessories that provide accurate and rapid column switching and LC×LC (comprehensive LC) capabilities. For LC×LC, acceptance has also been brought about by the availability of more orthogonal stationary phases and rapid second-dimension column configurations such as short, fast superficially porous particle (SPP) columns and, to a lesser extent, monolithic columns. The 2D techniques are mainly useful when complex samples are encountered. Nevertheless, Professor Peter Schoenmakers of the University of Amsterdam, The Netherlands, pointed out in his lecture that the technology still has a ways to go. First, method development is complicated, even more so than for LC–MS. It requires time and knowledge to become comfortable with the methodology. The incompatibility of the first and second dimensions can cause problems, especially with the injection of strong solvent from the first dimension. The direction of his group's research is to make the technique easier to use by addressing some of the practical issues. Normally, a loop is used to capture eluent from the first dimension (referred to as "passive modulation") but work by his research group has switched to a packed column (trap) instead where analytes can be concentrated by adsorption and eluted with a solvent that is more compatible with the second dimension ("active modulation"). This specific case of active modulation is called stationary phase–assisted active modulation. In this mode, it is possible to remove salts from the first dimension so that they do not affect the second dimension and possibly enter an MS detector. To aid in the development of operational parameters, the group has developed an optimization program that yields high peak capacities with short analysis times, a great help in method development. At the end of his lecture, Professor Schoenmakers introduced the principle of 3D separations that might be used to tackle extremely complex samples.
Another form of active modulation in 2D-LC is active solvent modulation (ASM). Xiaoli Wang of Agilent Technologies addressed the solvent strength compatibility problem in the concept of ASM by introducing a weaker solvent into the output of the first dimension so that the analytes can be more focused on the second-dimension column preventing peak distortion, loss of resolution, and lower sensitivity. The researchers evaluated four approaches to implement ASM:
1. An extra pump to dilute sample from first dimension
2. A partial loop fill to decrease amount of noncompatible solvent
3. Fixed solvent modulation
4. ASM
Overall, the first approach was ruled out because of the extra expense of adding another pump to the LC system.
The second approach involved using a modified second-dimension gradient with weak solvent at the start and sandwich sample with low percent organic on both sides. It was found that this approach was not acceptable for very hydrophilic compounds, but gave good peak shape for hydrophobic ones. The fixed solvent modulation approach involved a plumbing change such that the sample from the first dimension was diluted with the second dimension mobile phase. However, using a gradient split for the second-dimension pump causes a baseline disturbance that was deemed unacceptable. Finally, ASM involving a built-in bypass internal to the 2D switching valve proved to give the best results with no baseline disturbance and best peak shape. The results obtained from coupling HILIC with reversed-phase chromatography demonstrated that this approach was optimal.
In the pharmaceutical laboratory, 2D-LC has been gaining in popularity, not so much in the comprehensive mode where every fraction from the first dimension is directed to the second dimension (as inferred above) but more for heart cutting (either single or multiple fractions) when performing impurity profiling. In this approach, the second dimension can be decoupled from the first dimension and the second dimension is optimized for the best resolution of impurities. Jessica Lin and colleagues at Genentech presented a lecture on the quantitative aspects of 2D-LC. Much of the earlier work has been devoted to the qualitative aspects, while in the pharmaceutical industry, especially in the quality control (QC) lab, quantitation is more important. In her work, she compared the quantitative performance of single-heart cutting (SHC), where a single fraction is collected across a peak of interest, with multiple heart cutting (MHC), where multiple fractions are collected across a peak. Parameters such as sample loop size, second-dimension column geometry, and loop fill percentage (lower organic content for less filling) were studied to determine their effect on peak width, peak shape, and overall resolution. A method validation was conducted and quantitation performance, including precision, linearity, accuracy, and sensitivity, was compared between SHC and MHC. This study provided guidance for accurate impurity quantitation.
Microfluidics
The area of microfluidics has long fascinated researchers in liquid-phase separations. By reducing the size of the separation system and the volume of the sample and reagents, along with the ability to interface the separation technology to the detector requirements, especially for mass spectrometry, miniaturization brings many advantages to the laboratory. Microfluidics has been around for a long time, even resulting in some commercial systems, the so-called lab-on-a-chip devices. However, miniaturized systems have never been thoroughly accepted by the user community and the technique still languishes in the research laboratory. Nevertheless, very interesting work, especially in academia, may help to elevate microfluidics technology to the forefront.
In his plenary lecture, after receiving the 2017 CASSS Award for Outstanding Achievements in Separation Science, Professor Robert Kennedy of the University of Michigan presented his work using microchip electrophoresis for protein analysis related to high-throughput screening (HTS) and western blotting. His work in the separation of protein–protein complexes, which are involved in intracellular signaling to generate quantitative information on binding and selectivity, is quite impressive. His laboratory has also demonstrated fast (less than a second) separation of substrates and products from enzymes, which facilitates rapid enzyme analysis in which a droplet microfluidic system allows discrete samples to be rapidly loaded in sequence onto a chip for injection and separation. Throughputs of 1000 injections in 17 min gave very reproducible results, suggesting that this approach may open the door to robust HTS methods.
In the same opening plenary session, Professor Takehiko Kitamori of the University of Tokyo, Japan, presented a futuristic lecture on microfluidics and especially nanofluidics as promising platforms for femtoliter bioanalysis. The latter dimensions are three orders of magnitude smaller than microfluidics. For many years, his research has focused on micro- and nanofluidic applications in a wide variety of areas, including wet analysis, cell bioassay, immunoassay, and chemical synthesis. The group has pioneered the use of thermal lens microscopy for continuous measurement readout. Some applications in the sample prep and chromatography areas include femtoliter liquid–liquid extraction, femtoliter volumetric pipetting, and attoliter chromatography with gradient generation. He was able to detect only 46 molecules in an 11-fL volume and is now investigating single cell–single molecule analysis.
On the practical side of microfluidics, Professor Michael Breadmore of the University of Tasmania, Australia, reported on the status of 3D printing microchemical devices, both in a tutorial and an oral presentation. The discussion about 3D printing is no longer about whether it can it be done, but how can it be used in the laboratory to fabricate devices that may prove useful in analytical chemistry, especially in microfluidics and potentially in LC column production.
In microfluidics, lithography microfabrication is currently the most popular approach to building microchannels in media such as glass or plastic. However, making a microchip by this process is time-consuming and not suitable for high-speed fabrication. A variety of 3D printers available on the market can be used to construct microfluidic devices in a matter of hours, sometimes minutes depending on the complexity. Dr. Breadmore compared three printers with the best potential (see Figure 2): Inkjet/PolyJet, fused deposition molding (FDM), and digital light processing–stereolithography (DLP-SLA). The resolution of all three of these printer types do not quite meet the needs of very small channel fabrication with 25–50 µm being a typical X-Y-Z resolution for these printers. Nevertheless, successful microfluidic devices have been constructed in their laboratory using various polymeric materials (acrylonitrile butadiene styrene [ABS], polylactic plastic [PLA], poly[methyl-methacrylate] [PMMA], and acrylic-epoxy). Each printer has its advantages and disadvantages. For example, the FDM printers are easy to use, are inexpensive, allow a broad selection of materials, and enable multiple materials to be printed together to allow for multifunctional devices. One can embed reagents or other components into the system since the printer allows users to pause and stop operation. On the other hand, the FDM printers show low resolution, give a rough printing surface, and have low repeatability. PolyJet printers also can handle multiple materials (current ones up to six), provide a better surface finish, have robust hardware and software, and receive professional service from manufacturers. The negatives are their cost (purchase and running), the postprocessing time they require (days), their support material problems (removal from channels), the surface roughness they yield, and their poor microfabrication (<200 µm). The DLP-SLA printer provides the best X-Y-Z resolution, good laminar flow in channels (which is important in microfluidics), is the fastest but has fewer materials to work with, uses large amounts of resin, and has poor scalability. So, Dr. Breadmore could not recommend one type over the other since the choice is dependent on the needs of the worker. Figure 2 shows the result of fabrication of a simple microfluidic flow system design output of three different types of 3D printers (FDM, Polyjet, and DLP-SLA), with two print orientations using the FDM printer. All designs have exactly the same internal fluidic design (inlet diameter: 500 µm; outlet diameter: 750 µm). The geometry for channels is 1:1.5 for all devices, with a 40 mm × 20 mm footprint to fit all printer build spaces. Clearly, 3D printing should have a great future with its potential for rapid prototyping, ease and potential speed of manufacturing, and ability to design complex systems.
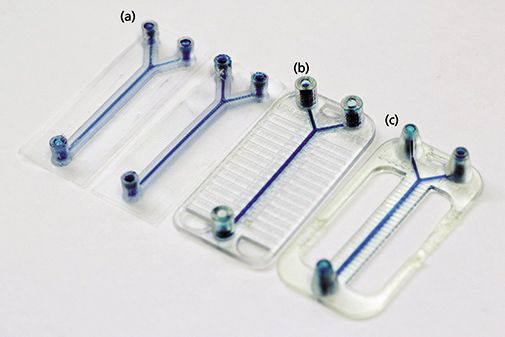
Figure 2: Typical microfluidic fabrications using three types of 3D printers: (a) FDM (two printing alignments), (b) PolyJet, and (c) DLP-SLA. (Courtesy of Niall McDonald, Feng Li, and Michael Breadmore, University of Tasmania, Hobart, Australia.)
Conclusion
Based on the popularity of the Australasian version of the HPLC series, it will continue into the future. The 49th Symposium will return to Kyoto, Japan, and will be chaired by Professor Koji Otsuka of Kyoto University; the dates will be December 1–5, 2019 (hplc2019kyoto.com).
References
(1) N.P. Macdonald, J.M. Cabot, P. Smejkal, R.M. Guijt, B. Paull, and M.C. Breadmore, Anal. Chem. 89, 3858-3866 (2017).
Ronald E. Majors is an analytical consultant with ChromPrep in West Chester, Pennsylvania. Please direct correspondence to: ronald.e.majors@gmail.com

Reversed-Phases for LC Deliberately Doped with Positive Charge: Tips and Tricks for Effective Use
May 13th 2025In this month's edition of LC Troubleshooting, Dwight Stoll and his fellow researchers discuss both the benefits (improved peak shape/loading) and challenges (excessive interaction) associated with charge-doped reversed-phase (RP) columns for both analytical and preparative separations.
Evaluating the Accuracy of Mass Spectrometry Spectral Databases
May 12th 2025Mass spectrometry (MS) can be effective in identifying unknown compounds, though this can be complicated if spectra is outside of known databases. Researchers aimed to test MS databases using electron–ionization (EI)–MS.
Discovering the Hidden Plastic in Garden Compost with Pyr-GC–MS
May 12th 2025An Australian study used pyrolysis coupled to gas chromatography-mass spectrometry (Pyr-GC–MS) to analyze the presence of plastic polymers in commercial and homemade composts. LCGC International spoke to Simran Kaur—a PhD candidate at the Queensland Alliance for Environmental Health Sciences (QAEHS) at The University of Queensland in Woolloongabba, Australia—to find out more about her team’s findings.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)