Highlights of HPLC 2006
LCGC North America
The popular HPLC 2006 Symposium was held in June in San Francisco, California. It is the premier annual meeting in liquid phase separations technology.
The 30th International Symposium on High Performance Liquid Phase Separations and Related Techniques, which alternates between Europe and North America, was held, for the third time, in San Francisco, California, June 17–22, 2006. More affectionately known as HPLC 2006, the symposium is the premier scientific event for bringing together the myriad techniques related to separations in liquid and supercritical fluid media. Chaired by Dr. John Frenz of Genentech (South San Francisco, California), and managed by Barr Enterprises (Walkersville, Maryland), HPLC 2006 assembled over 1200 scientists from a total of 45 countries. This number included vendor representatives from over 100 exhibitors for the three-day instrument, software, and consumables exhibition, conferees, one-day registrants, exhibit-only registrants, and press.

Ronald E. Majors
The five-day-plus event had a total of 680 symposium papers, 160 oral sessions ( many presented during simultaneous sessions), 520 posters in sessions with 27 themes, and a total of seven vendor seminars. Thus, attendees had their hands full deciding how to allocate their time. With an ample social event schedule and seven short courses held during the previous weekend, most attendees who stayed for the week were ready to return home to relax. The short course topics reflected areas of current interest and included validation, advanced high performance liquid chromatography (HPLC) method development, HPLC troubleshooting, enantiomeric separations,biomarkers, solid-phase extraction (SPE), and process separations.
This year's event again featured a special program for scientists under the age of 35. Of the 17 lectures presented, surprisingly, a third of them were female scientists with Norma M. Scully of University College (Cork, Ireland) achieving the top lecture award, as voted by the conferees, with her talk entitled "Supercritical Fluid Generated Stationary Phases for Liquid Chromatography and Capillary Electrochromatography." Using supercritical carbon dioxide as a solvent for the preparation and packing, she was able to achieve better than 117,000 plates/m with an average asymmetry factor of 1.07 for a monomeric C18 phase. At the opening ceremony, a number of travel scholarships were given to deserving students including the Halasz Foundation scholarships. All students who applied for these travel grants were provided funding to help defray the costs of attending this meeting.

Table I: HPLC 2006 papers presented by technology or technique
Obviously, HPLC was the predominant technology used by presenters in the technical sessions at the symposium. Compiled after a perusal of the poster and oral presentation abstracts, Table I provides a rough breakdown of the coverage of liquid phase technology and techniques in the separation sciences. Table II is a similar breakdown for application areas. This year, I also tabulated the detection principles (Table III) that were used. Not every abstract indicated the detector or column used, so only those that provided this information was counted. The category assignments were based upon the main emphasis of a particular scientific paper as well as separation and detection techniques used.
In this installment of "Column Watch," I will present some of the scientific highlights of HPLC 2006. This report will emphasize column developments; it was the most active area of development at the meeting. Because it was virtually impossible for one person to adequately cover all oral and poster papers, I obtained summaries from some of my colleagues, who are acknowledged at the end of this paper.

Table II: Presented by application area
Poster Sessions
The mainstay of HPLC 2006 was the poster sessions, where more detailed applications and methodology studies were reported, often in very specific areas, and face-to-face discussions with the authors were conducted. Fortunately, many of the poster authors were kind enough to provide small reproductions of their poster papers that could be taken for later perusal. Some collected business cards and addresses for sending poster reprints by mail or e-mail. This year, all posters were up for all four days allowing participants to adequately cover these sessions. Although the poster format was smaller (4 ft × 4 ft) than previous years, there were only minimal complaints about the new size. By staggering the days that the posters were staffed by presenters, poster sessions did not appear to be crowded. Posters were spread out a bit so some detective work was needed to find all the poster boards.
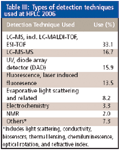
Table III: Types of detection techniques used at HPLC 2006
This year I once again had the privilege of leading the Poster Committee that chooses the top three posters at the symposium. With over 520 posters to cover, poster review was a challenging task. Fortunately, I had the able assistance of 33 members of the Poster Committee, who devoted a great deal of time and worked very hard to narrow down the huge collection of posters and helped to select the three winners by the end of the fourth day of the symposium. I want to recognize them individually by providing their names and affiliations in Table IV. Overall, the committee felt that the quality of posters was again outstanding and showed continued improvement over previous symposia in this series.
The Best Poster Awards, sponsored by Agilent Technologies (Wilmington, Delaware) were announced at the Closing Session. The three winners received Amazon.com gift checks. This year's winning poster was entitled "Preparation of Highly Efficient Monolithic Silica Capillary Columns for Separation of Highly Polar Compounds" and the coauthors were Kanta Horie, N. Saad, T. Ikegami, O. Fiehn, N. Tanaka (Kyoto Institute of Technology, Kyoto, Japan and University of California, Davis, California).

Table IV: Poster committee
In their paper, the authors showed how they were able to modify the surface of a silica monolith with acrylic acid functionality via an anchor group. This polar phase showed high permeability and good separation efficiency in the ion exchange and hydrophilic interaction chromatography (HILIC) modes. It was useful for polar compounds such as nucleobases, carbohydrate derivatives, and peptides. The authors also explored this novel capillary column as an orthogonal phase for multidimensional HPLC separations.
The winner of the second best poster, entitled "Glycomic Profiling of Blood Serum Derived from Advanced Breast Cancer Patients" were coauthors Zuzana Kyselova, Y. Mechref, P. Kang, R. Hickey, and M. Novotny of the METACyt Biochemical Analysis Center, National Center for Glycomics and Glycoproteomics and the Department of Medicine, Department of Chemistry, Indiana University (Indianapolis, Indiana). Their poster covered their work on the study of glycosylated proteins in blood sera that can be indicators of molecular changes that can occur during malignant transformation of normal cells to cancer cells. Using enzymatic release of glycans followed by derivatization by a capillary permethylation technique and MALDI-TOF-TOF mass spectrometry (MS), the authors investigated glycomic profiles of cancer patients and observed relative intensity changes in glycans as breast cancer developed and can prove to be a way to evaluate individual response of treated patients to various therapies.
The winner of the third best poster, entitled "The Effect of Particle Size Distribution on Flow Resistance and Band Broadening in Columns Packed with Sub-Two Micron Particles" were coauthors Jeroen Billen, P. Gzil, F. Lynen, P. Sandra, P. van der Meeren, and G. Desmet from the Pfizer Analytical Research Center, Vrije Universiteit Brussel, Belgium, and the University of Ghent, Belgium. This work investigated the HPLC packing particle size distribution on chromatographic performance and column backpressure of the new sub-2-μm materials. Using commercial packing simulator software, the authors were able to generate simulated packings and export information about them to a computational fluid dynamics program to calculate permeability and band broadening. Using actual commercial packings, the authors were able to study band broadening and flow resistance and compare the results to theory.
Photographs of two of the three winners receiving their prizes are shown in Figure 1. The third author was unable to attend the closing session and her portrait is shown alone.

Figure 1
New Column Technology Highlights
As seen in Table I and similar to the last six years of HPLC Symposia coverage (1–6), the development and study of columns and stationary phases still dominates the new technologies. At least seven oral sessions and an equivalent number of poster sessions were devoted to column technology, retention mechanisms, high-throughput columns, ultrahigh-pressure HPLC, and the like. If one combines all papers pertaining to column technology, about 30% of the presentations at HPLC 2006 covered this topic. Despite all the advances made in column technology to date, investigations on further developments in academia as well as the commercial side still are taking place.
From what I observed at HPLC 2006, the current "hottest topic" in HPLC column technology appears to be which of the two alternative approaches to developing faster separations and generating more column efficiency will be favored in the long run. Both approaches work on optimizing the phase ratio along with balancing column permeability. One approach is the monolith camp, where improvements in the homogeneity of the continuous beds and improvements in efficiency by adjusting the relative sizes of the macro- and meso-pores, which can be varied independently using the sol-gel synthesis process, has led to better overall chromatographic columns. By varying this ratio, monolith permeability can be affected, sometimes in a detrimental way.
The packed column camp has used conventional spherical silica particles but with sub-2-μm particles sizes being favored, each having various particle size distributions. The short columns, usually less than 50 mm in length, are run at high linear velocities, giving high throughput. On the other hand, if more theoretical plates are required, then these small particles are packed into longer columns (as long as 15 cm), but when these columns are run at higher flow velocities, more back pressure is generated. Thus, the use of high column temperatures is now fashionable. Higher operating temperatures reduce the column back pressure due to lowering the mobile phase viscosity. A side benefit is more rapid solute mass transfer to improve the column efficiency.
Monolith Column Technology: At HPLC 2006, monolithic phases dominated columns' technology and applications' papers with 40% of column presentations devoted to this technology. Overall, more emphasis was devoted to the preparation of monoliths in capillaries and in microchannel chip-based systems than to columns of conventional dimensions.
Monolith columns have been desirable because they exhibit high permeability–low pressure drop (due to increased bed porosity), show good separation efficiency, have the absence of frits to confine the packing material, are easy of fabricate, and can be made fairly reproducibly. Although this technology has been around for several years, as a routine tool it has yet to see widespread acceptance. But with continued academic advances reported at HPLC 2006, if commercialized, users should take more advantage of these columns. Reports were devoted to improving both polymer- and silica-based monoliths. Not surprisingly, more work is being performed on polymeric monoliths because silica monoliths generally are governed by patent protection, so further commercial development has been stymied. Overall observations indicate that silica-based monoliths seem to work best for small molecules while the polymeric monoliths are best for macromolecules. Both theoretical and practical studies have been applied to understanding how to increase phase ratios and homogeneity of the co-continuous structures of silica skeletons.
Major practical advances in silica-based monolith technology occurred in the studies of Tanaka and coworkers from the Kyoto Institute of Technology, Japan. These workers were able to increase monolith homogeneity as well as the phase ratio in capillary columns that resulted in smaller through-pore sizes than those of previous preparation, which in turn gave better column efficiency with columns now equivalent to packed columns of 1.5–2 μm silica particles. With the smaller through-pore sizes, the permeability was generally lower than the previous larger through-pore sizes but the pressure drop is still less than experienced with packed silica particles of the same column dimensions operated at the same linear velocity generating the same efficiency. The increase in the amount of silica and mesopores contributed to an increase in solute retention by more than 70%. If the performance shown on these monolithic columns can be put into practice on a commercial basis, the new sub-2-μm particulate columns will have some stiff competition.
In several papers, silica monoliths were further functionalized by the incorporation of polar functionalities into their backbone. One such modification was cited earlier in the winning poster from Tanaka's group, but other capillary columns were prepared by the on-column polymerization of acrylamide on a monolithic silica capillary column modified with N-(3-trimethoxysilylpropyl)methacrylamide as anchor groups. This method of modification of monolithic silica resulted in a stationary phase for the HILIC mode. In a similar manner, hybrid silica monoliths can be further functionalized using octadecyl methacrylate via free-radical polymerization and then used for capillary reversed-phase chromatography on 25 cm × 200 μm columns. Polystyrene-divinylbenzene monoliths were functionalized with cation exchange groups and packed into 75-μm i.d. nanocolumns for proteomics investigations.
Most polymeric monoliths reported in San Francisco were either polymethacrylate- or polystyrene–divinylbenzene (PS-DVB)-based-chemistries, sometimes as copolymers. Most of these monoliths can be modified by subsequent chemical reactions. Methacrylate polymeric monoliths with affinity ligands based upon boronic functionality for both LC and capillary electrochromatography (CEC) were the subject of several papers. These phases are useful for retaining ribonucleosides and catecholamines while monolithic capillary columns having immobilized lectins or oligosaccharides achieve separations of sugar-binding proteins and glycoconjugates. Polymethacrylate-based monolithic matrices modified by simple radically initiated copolymerization using polyethylene glycol (PEG) functional monomer and a hydrophilic cross-linked resulted in affinity monoliths that had low, nonspecific adsorption and could be used to isolate targeted proteins. In a lecture, Milton Lee of Brigham Young University (Provo, Utah) discussed his group's research on cross-linked polymer-based monoliths, one of which used polyethylene glycol diacrylate (PEGDA) as a crosslinker. They used polymeric monoliths due to their better pH stability than silica. The monolith that was formed using this PEGDA crosslinker provided a very biocompatible polymer which could be used as an HPLC packing, a protein precipitation agent that does not denature, a sieving matrix for capillary gel electrophoresis and several other uses. Anion-exchange functionality could be grafted or incorporated with copolymerization directly. Cation-exchange functional groups also were incorporated.
Another high-performance epoxy polymer-based monolithic capillary column for mainly small molecule separations was prepared using an epoxy monomer with amines. Synthesis by simple heat-induced polymerization in an appropriate porogenic solvent afforded really homogeneous co-continuous monolithic structure having submicrometer-size skeletons with micrometer-size through pores, which was applied to nucleosides. Chiral moieties also have been incorporated into a monolithic structure using chiral epoxy chemistry. Latex-coated monoliths provided very fast separations in ion chromatography.
Homemade and commercial monoliths are starting to see a variety of applications. Monoliths in the capillary format with advanced detection techniques such as LC–MS–MS and MALDI-TOF MS–nuclear magnetic resonance (NMR) spectroscopy have been applied to solving problems in proteomics and other biorelated disciplines. Some other applications examples noted in posters at HPLC 2006 included uranium in sea water, sugar phosphates, peptide sequencing, separations of oligonucleotides, racemic alcohols, various pharmaceuticals including pilocarpines, zinc pyrithione, glimepridine, inorganic anions and cations, chiral compounds, and enzymes. Monoliths have been used as solid-phase extraction devices for phenols in tap water and packed into micropipette tips containing immobilized trypsin for rapid peptide digestions. Monoliths as the second dimension column could provide an answer to the speed mismatch problems with comprehensive 2-D HPLC systems.
Commercial silica monoliths (Chromolith from Merck KgAa, Darmstadt, Germany) have been available in 4.6 mm and larger internal diameters and 100-mm lengths. For these columns to provide high-speed separations, they must be run at fairly high flow rates, sometimes as high as 3 or 4 mL/min. Such flow rates are well beyond those desired by mass spectroscopists, who generally prefer 2.1-mm particulate columns that run at flow rates of 0.4–0.6 mL/min. Merck has responded and at HPLC 2006, Karin Cabrera discussed their latest addition of 3.0-mm columns, not quite what the MS people desire but closer. Apparently, fabrication of 2.0- or 2.1-mm silica monoliths are not as easy as first imagined because during the manufacturing process, the solid silica rods must shrink to the desired outer diameter and then must be encased in a PEEK housing.
Continuous polymer monoliths can be fabricated into various physical formats. At HPLC 2006, a report on the successful synthesis of porous monoliths in 50-μm thick sheets for thin-layer chromatography (TLC) might be of interest. Such TLC sheets contain no binding agent, which implies that they can provide better separations. Probably the most interesting new format for monolithic columns was the successful fabrication of porous layer open tubular (PLOT) columns with diameters of 5, 20, and 50 μm. For years, theoreticians have postulated that tiny open tubular columns ultimately could provide the best operating efficiency but the thin film of stationary phase in these nanocolumns had almost no sample capacity. Capillary GC columns have used porous layer open tubular (PLOT) designs phases for years, but no one had applied them to separations in the LC mode. . These tiny diameter PLOT columns were applied to protein separations to provide an efficient, sensitive separation technique, often required in these studies.
Although polymeric disks have been around for some time, the development of silica-monolith disks of around 3.1-mm diameter that can be inserted into standard membrane filter holders was reported at the symposium. Disks with C4, C8, and C18 stationary phases could be stacked to provide multimodal separations whose efficiency was based upon the number of stacked disks (that is, column length). Further reports on the synthesis of monoliths inside of microfluidic channels in chips implies that this can become the favored approach for microfluidic separation devices. Packing particles into nanoliter channels in chip-based systems has proven to be a challenge.
Besides silica- and polymeric-based monoliths, multiple reports of titiania-based monoliths as well as papers on hafnia-based monoliths and inorganic-organic hybrid-monoliths showed that other chemistries are applicable to these high permeability columns. Silica-based monoliths have the same high-pH limitations of silica particles and these other phases might prove more suitable for high-pH work.
Sub-2-μm Columns: The second most covered topic, with 20% of the papers, was the study, development, and applications of sub-2-μm columns. Compared with monoliths, the sub-2-μm particles are the normal progression of the decrease in packed column particle sizes over the years from 10 μm in the 1970s to 5 μm in the 1980s and 1990s to the 3–3.5 μm particles in the 1990s and 2000s. Mean particle sizes of 1.5, 1.7, 1.8, 1.9, and 2.0 μm were discussed, mostly in commercial presentations. In one poster paper, delivered by M. Takahashi and coworkers from Shimadzu (Columbia, Maryland), the merits of 2.2-μm reversed-phase particles were discussed, in which the pressure drop will be somewhat lower than with the sub-2-μm particles. Their approach was to treat high-throughput requirements for the entire HPLC system, not only the column itself, where cycle time must be considered as part of the argument. System elements such as cycle times of the autosampler and gradient column equilibration combined with high temperature operation (80 °C) can sometimes lead to results achieved by sub-2-μm columns. One paper discussed the alternating column regeneration principle as helping overall cycle time in a high-throughput environment. In this approach, two columns are employed where one column is performing the analysis while the other column is undergoing regeneration. When the analysis on the first column is finished, the freshly reequilibrated second column is ready for the next analysis.
Several practical and theoretical papers discussed the merits and disadvantages of narrow, wide, and engineered particle size distributions with the sub-2-μm particles. It was concluded that more work on modeling the particle size distributions is required and that frictional heating of the solvent generated at higher flow rates at higher pressure can cause data misinterpretation.
Joining the silica-based reversed-phase sub-2-μm columns were nonporous ion exchangers with pore sizes of 300 and 500 Å for biomolecules. These new phases were based upon highly cross-linked PS-DVB particles that were tested to withstand pressures of 8000 psi.
There were many applications examples shown where HPLC methods performed on older 3- and 5-μm columns were easily converted to the sub-2-μm columns with greatly reduced analysis times but with equivalent results, although one author found that limits of quantitation (LOQ) at the 0.05% level relative to a main component was hard to achieve using them. Applications to trace enrichment, column switching, impurity and stability (degradation) studies of pharmaceuticals, and drug metabolites were shown. Some discussions of frictional heating and column and packing stability at high pressures were initiated, as well as instrumentation issues such as extracolumn effects, gradient delay volumes, and data rates. The high speeds shown with short sub-2-μm columns also can prove useful as the second column set for comprehensive- and two-dimensional LC. In his plenary lecture during the closing session, Professor Pat Sandra of the University of Ghent gave an interesting lecture in which he discussed all elements of the successful use of sub-2-μm columns at elevated temperatures. He concluded that with the sub-2-μm column coupled to a modern high-pressure liquid chromatograph that LC is now showing results typical of capillary GC. Figure 2 shows his example of the analysis of furocoumarins in a lemon and orange oil extract that demonstrates a peak capacity of greater than 170, an average peak width (at 4 sigma) of less than 0.12 min, and a separation time of under 20 min with GC-like resolution.
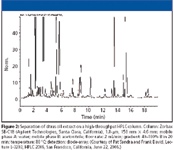
Figure 2
Chiral separations: Chiral chromatography was another relatively hot area of activity. Ever since the U.S. Food and Drug Administration declared that certain drugs with a chiral center should be enantiomerically pure, chiral separations have been receiving increased attention. Chiral compounds can be separated by HPLC, capillary electrophoresis (CE) and other electrodriven technologies, and supercritical fluid chromatography (SFC). SFC has found a unique niche in the preparation of pure enantiomers on a large-scale basis. Several papers covered various aspects of SFC chiral separations including recycle chromatography, in which several short chiral columns are used in series and then unresolved enantiomers are refed into the same column set until resolution is achieved. From what I can tell from papers at HPLC 2006, for the HPLC approach, chiral column selection still involves scouting multiple chiral stationary phases until one is found to resolve the sample. One paper described a 10-port valve setup that could be automated to perform the scouting experiment more conveniently. The use of selective chiral detection such as optical rotary dispersion and a new proprietary technique termed Magneto-Optical Enantiomeric detection (MOPED, Stheno Corporation, Atlanta, Georgia) can aid in identification of chiral purity.
An interesting concept in chiral packing technology was presented by Benjamin Chaloner-Gill of Stheno and coworkers from Evolved Nanomaterial Sciences, Inc. (ENS) (Cambridge, Massachusetts). While most chiral packings involve a chemical mechanism of "three-point interaction," where various functional groups on an enantiomer will interact with the chiral stationary phase by π–π, hydrogen bonding, and other interactions, their polymeric material, called ESP, separates on a physical basis by the shape of the chiral compound. ENS' polymers self-assemble into scaffolds with various channels and pores along the structure, some as small as 11-nm wide. The walls of the channels are studded with receptors; as chirals are forced through the channels, the receptors bind to the desired enantiomer while the remainder washes away. By changing the makeup of the polymers involved, the scaffolds separate specific chirals as needed. Exactly how the company can tune its polymers to create structures and pores of specific nanoscale dimensions is proprietary and remains a mystery, but the fundamental approach — catching chirals in a net rather than separating them chemically — could pose some interesting separation possibilities.
Other new stationary phases: I made some observations at HPLC 2006 in terms of new stationary phase investigations. Researchers now seem to be developing phases that are useful outside of the regular modes of reversed phase, ion exchange, size exclusion, and normal phase. Separation modes that were unknown (or unused) a few years ago are starting to appear in publications — hydrophilic interaction chromatography, mixed-mode separations, aqueous normal phase, ion exclusion, and separations based upon physical forces (not chemical) to name a few. I have selected a few examples of some of these newer modes to illustrate this phenomenon.
For example, Joseph Pesek and coworker Maria Matyska (California State University, San Jose, California) described further work on a stationary phase where hydride groups (Si-H) have replaced most of the silanols (Si-OH). The surface of hydride-based stationary phases creates a separation medium that possesses unique properties when compared with ordinary silica. One such feature is the ability to utilize mobile phases ranging from 100% aqueous to completely organic for the analysis of ionic–polar compounds as well as solutes that are highly hydrophobic. A single phase with a bonded organic moiety can be used with three modes of separation: reversed phase, aqueous normal phase, and organic normal phase (that is, compounds with polar–ionic functional groups are retained in mobile phases such as hexane–ethyl acetate). For hydride materials in the aqueous normal phase, bases are retained under acidic conditions in high organic content mobile phases, so it is not necessary to use high-pH eluents.
Although mixed-mode stationary phases normally are not desired in HPLC, Michael Laemmerhofer of the University of Vienna, Austria, used them to his advantage when developing a column for peptide separations. A mixed mode (reversed-phase and weak anion exchanger) was bonded to silica. His approach employed a long hydrocarbon chain with an embedded sulfur group plus a nitrogen-containing end group (2 nitrogens are part of a 2.2.2-bicyclic octane, one nitrogen is at the bridging position and one is on the connecting side chain in the 3-position). By variation of the isocratic or gradient mobile phase, conditions using aqueous 10-mM triethylammonium phosphate and acetonitrile, he was able to separate peptides by hydrophobic interaction, hydrophilic interaction, reversed-phase, anion-exchange, or ion-exclusion chromatography. Changes in elution order were observed as the composition of the mobile phase was changed, which helped in separating closely related peptides.
Other observations were based upon the large number of nonclassical approaches to packing synthesis for reasons of lack of stability and ruggedness or new and different selectivities compared with the classical stationary phases. Titania, zirconia, carbon-coated silica, and polymeric fibrous materials are just a few examples of new base materials. For example, César Silva and coworkers from the State University of Campinas (Sao Paulo, Brazil) have extended their work on the preparation of titanized and zirconized silicas modified with polymeric-type C18, including one containing embedded polar (carbamate) groups to help maintain symmetric peaks for basic compounds. The Engelhardt test revealed significant peak asymmetry using acetonitrile–water mobile phases, ameliorated by using a phosphate-buffered mobile phase. Stability tests showed that all metalized phases were more stable than similar phases prepared on the same silica without metallization, while zirconized-silica support produced phases that were more stable than those prepared on the titanized-silica support.
To make higher-pH bonded phase silica, a group from Restek (Bellefonte, Pennsylvania) increased the stability of the silica particle by adding a polycarbosilane barrier layer to protect the particle from aggressive basic or acidic conditions. This layer, with multiple points of attachment to the silica particle, yields a more robust, modified, underlying surface, which was then further reacted to provide the working functionality group such as C18.
Kenneth Markus and coworkers from Clemson University (Clemson, South Carolina) updated last year's presentation on capillary-channeled polymer fibers as a packing material for HPLC. These fibers display high wicking action and a higher surface area than traditional fibers. In this study, branched polypropylene, polyester, and nylon fibers were studied and appear to work best with large molecules like proteins. The different polymers permit the use of different pH and mobile phases and typical pH and ionic strength gradient. They can be operated at a higher flow rate than typical protein columns and display very low back pressures. Markus suggested that short, fiber-filled capillaries would be a good second choice for comprehensive LC×LC experiments. In a separate poster, the same group used the fiber columns as adsorptive materials for the SPE of proteins from salt, low molecular weight organic buffer, and detergent-containing solutions. In this case, the polymer capillaries were affixed to micropipette tips.
Retention Mechanisms and Method Development
Many of the theoretical presentations had practical consequences. Georges Guiochon of the University of Tennessee (Knoxville, Tennessee) presented a keynote entitled "Retention Mechanisms in RPLC are Still More Complex Than We Thought: New Data, New Mysteries," in which he continued his studies of nonlinear isotherms observed for the retention of polar basic and acidic analytes on reversed-phase packings of different bonding densities. Using frontal analysis over a wide concentration range (104 ), he showed that for a typical C18 reversed-phase packing, there are as many as three types of sites — high-energy sites, low energy sites, and supersites (residual silanols?) existing simultaneously, which Guiochon referred to as adsorption energy distribution. These different sites can be observed by nonlinear chromatography, while linear chromatography gives a single data point of maximum retention at intermediate bonding densities. The high-energy sites, probably resulting from analyte partitioning into the C18 phase, mainly control retention. Unfortunately, their density is low, hence, the saturation capacity is small compared with the surface area of the packing. In the 0–60% methanol range, the energy and density of the high-energy sites are sensitive to the organic solvent content while properties of the low energy sites are not. The presence of buffer also influences adsorption isotherms. Because most reversed-phase LC packings are heterogeneous with high-, low-, and super- energy sites, retention mechanisms are usually mixed, which makes it difficult to compare different packings. Guiochon went on to explain that the energetic inhomogeneity of the surface explains the poor loadability of basic compounds such as nortriptyline.
David McCalley of the University of the West of England (Bristol, UK) followed up with a talk on overloading of ionized solutes on highly inert, reversed-phase LC columns where the contribution of ionized silanols appears to be minimal. Overloading can be observed for submicrogram quantities of charged bases on inert C18 phases of conventional dimensions, giving serious peak broadening and reduced solute retention. Solute overload also is reduced in the analytical range with increased buffer ionic strength. Ionic repulsion of solute ions of the same charge can explain many aspects of overloading behavior, including the similar overloading of purely polymeric phases that have a completely different structure to silica-C18. However, it fails to explain the existence of multiple overloading sites mentioned by Guiochon. He and his coauthors examined ways of minimizing these analytical overloading effects, including the influence of pH, the role of the organic modifier, and investigated the possible benefit of using mixed-mode reversed-phase columns to improve the loading capacity of the phase by provision of additional retention sites. A poster paper by Brian Bidlingmeyer (Agilent Technologies) agreed that comparative column tests should specify the loading factor, which has been neglected by most workers.
Heinz Engelhardt, in a session celebrating his retirement from the University of Saarland, Germany, reviewed the different reversed-phase stationary phases that show different properties due to residual silanols (as much as 50% of these are available) and to residual metal content (metals in the packing enhanced the acidity of residual silanols). Thermally treated and rehydrated silicas react better with silanizing reagents, while polar-embedded groups modify retention orders due to changes in hydrophobic interactions.
Applications Highlights
As can be seen in Table II, protein and peptide separations and proteomics again dominated liquid-phase applications. This year, the discovery of biomarkers, both large and small molecules, for disease diagnosis was a popular topic. Pharmaceutical companies are looking for tools that will be able to measure and predict the efficacy of candidate drugs in shorter times and with less expensive clinical trials. Disease-specific biomakers can speed up the process of drug discovery. For protein-based biomarkers, the use of multidimensional LC coupled with MS and MS–MS is getting to be a standard technology. With even more techniques now available for the removal of high-abundance proteins from human plasma, trace levels of proteins are more readily detectable, although some presenters noted that sometimes small concentrations of desired proteins might tag along with the high-abundant proteins that are removed.
As usual, separations of pharmaceuticals (bulk drugs, tablets and other formulations), including impurity profiling, had a strong showing with presentations from most of the major pharmaceutical companies. Furthermore, the analysis of drugs, drug metabolites and endogenous compounds in biological samples, and clinical chemistry continued to have a large following. Many poster presenters were involved in discussions with conferees about their experimental approaches to solve these problems.
The number of applications papers using CE and related techniques was quite strong again this year (Table I). This technique, which has slowed commercially, has been used in solving many problems in the life sciences, environmental science, metal analysis, and many other complex problems. At HPLC 2006, there were presentations of continuing studies of eliminating protein adsorption on capillary surfaces by various coating or noncovalent adsorption of hydrophilic polymers. Sample introduction for capillary systems always has been a problem, so several papers were devoted to using stacking techniques to help to concentrate trace samples. The use of nonaqueous CE for chiral compounds and other pharmaceuticals continues to grow. With two-dimensional separations now gaining favor, some workers reported the successful combination of CE or micellar electrokinetic chromatography with ion chromatography or reversed-phase chromatography. These two approaches were truly orthogonal in the mechanisms.
I was surprised at the lack of emphasis on CEC this year. At one time, CEC sessions filled the halls; at HPLC 2006, there were only a couple of oral presentations and a handful of poster papers that stressed this technique. Perhaps CEC has not lived up to its promise; for the most part, there have not been too many separations problems solved by CEC that could not be accomplished by HPLC or CE alone. It will be of interest to see if that "killer application" can be found in the future.
Detection Techniques
As can be seen in Table III, LC–MS and LC–MS-MS were again the dominant detection methods used in the oral and poster presentations at HPLC 2006. Almost 50% of all papers that provided information on detection techniques used some form of MS.
As might be expected, UV detection, especially diode-array spectophotometers, was the second favored detection technique, albeit a distance behind MS.
Evaporative light scattering detection (ELSD) has been gaining in popularity. This detector is universal, can be used with gradients, and can be more sensitive than refractive index for a variety of applications. But charged-aerosol detection based upon corona discharge can replace ELSD because it appears to be an order of magnitude more sensitive than ELSD. Charged aerosol detection is also an evaporative technique and is based upon the charging of aerosol-borne analytes by nitrogen gas and corona discharge with subsequent measurement of the charged nonvolatile analyte particles by a high sensitivity electrometer. In results provided in poster papers by ESA (Chelmsford, Massachusetts), LOD for carbohydrates of less than 10-ng on column with a dynamic range of four orders of magnitude and RSD values of less than 1.6% were typical. For lipid classes, low nanogram sensitivity was achieved. Charged aerosol detection's response is based upon a quadratic response, while ELSD has a polynomial response that varies with the amount of compound. Although charged aerosol detection is recommended for relatively nonvolatile compounds, a group from Astra Zeneca (Molndal, Sweden) found that even volatile compounds could be detected by the proper choice of mobile phase buffer.
HPLC 2007 is Next
The next major symposium in this series, the 31st International Symposium on High Performance Liquid Phase Separations and Related Techniques (HPLC 2007), moves back to Europe and will be held in Ghent, Belgium, June 17–21, 2007. This will be the first time this series has been held in Belgium, and co-chairmen of this upcoming event will be Prof. Jacque Crommen of the University of Liege (Liege, Belgium) and Prof. Patrick Sandra of the Research Institute for Chromatography (Kortrijk, Belgium) and the Pfizer Analytical Research Center (Ghent, Belgium). Figure 1d depicts the Symposium tradition of passing on a Swiss cowbell from the outgoing Chairperson to the new Chairperson. The very first HPLC meeting was held in Interlaken, Switzerland in 1973. For more information consult the official website at http://www.hplc2007.org/.
Acknowledgment
I would like to acknowledge the highlight contributions from some of the poster committee, my Agilent colleagues, especially Maureen Joseph and Bill Barber for loaning me their detailed notes, and to Carol Collins of the State University of Campinas (Sao Paulo, Brazil) for her summary of many of the columns talks that I was unable to attend.
"Column Watch" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgc-mag.com.
References
(1) R.E. Majors, LCGC 18(11), 1122–1134 (2000).
(2) R.E. Majors, LCGC 19(10), 1034–1048 (2001).
(3) R.E. Majors, LCGC 20(9), 830–841 (2002).
(4) R.E. Majors, LCGC 21(9), 872–887 (2003).
(5) R.E. Majors, LCGC 22(9), 870–882 (2004).
(6) R.E. Majors, LCGC 23(9), 989–1005 (2005)

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













