Hexavalent Chromium Determination Hamilton
The Application Notebook
Analysis of chromium species is made challenging due to the nature of the element and diverse sample matrices
Chromium (Cr) is a metal with an interesting relationship to the environment. Whereas trivalent chromium (Cr(III)) is an essential nutrient, hexavalent chromium (Cr(VI)) is a poison to humans and aquatic life and poses serious environmental and ecological threats. Recent studies of various ground and drinking water sources have detected toxic levels of Cr(VI). This dangerous trend has gained the attention of national and worldwide health organizations, such as the United States Environmental Protection Agency (EPA) and the US Food and Drug Administration (FDA), who seek to understand how widespread the problem is. The California Department of Public Health included Cr(VI) as an unregulated chemical requiring monitoring in 2001. Based on recent data, 3107 of 6565 public wells in Los Angeles, San Bernardino, and Fresno counties had Cr(VI) concentrations above 1 µg/L. A Public Health Goal of 0.02 µg/L was published in July 2011.
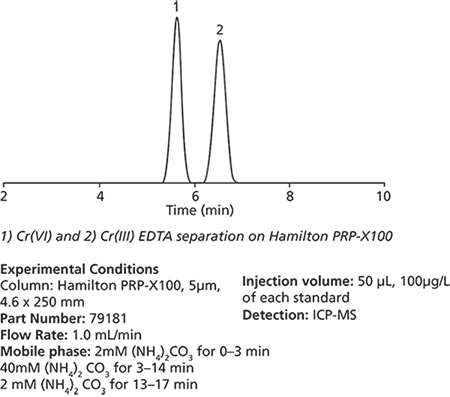
Analysis of chromium species is made challenging due to the nature of the element and diverse sample matrices. Because chromium exists in two oxidation states, it is important to differentiate between the nutrient, Cr(III), and the poison, Cr(VI) in samples. An HPLC–ICP-MS method using the Hamilton PRP-X100 has been developed in order to determine relative abundance of Cr(III) and Cr(VI) in diverse sample matrices.
Trivalent chromium (Cr(III)) is stabilized as a chelation complex by incubating the sample at 70 °C in 0.2 mM EDTA. The Cr(III)-EDTA complex is suitable for binding to an anion exchange resin. Resolution of the two species then becomes a straightforward isocratic separation. Although UV and conductivity are suitable for detecting chromium species, inductively coupled plasma-mass spectrometry (ICP-MS) is the method of choice for trace analysis.


Hamilton Company
4970 Energy Way, Reno, NV 89502
tel. (775) 858-3000, (800) 648-5950
Website: www.hamiltoncompany.com

Separation of Ultra-Short and Long Chain PFAS Compounds Using a Positive Charge Surface Column
December 11th 2024A separation of ultra-short and long chain PFAS (C1-C18) is performed on a HALO®PCS Phenyl-Hexyl column along with a HALO®PFAS Delay column which demonstrates excellent retention for both hydrophilic and hydrophobic analytes.














