Gas Cylinder Safety, Part 1: Hazards and Precautions
LCGC Europe
Many gas chromatographers are not fully aware of safe practices for handling high-pressure gas cylinders. Gas chromatography (GC) operators should be trained to properly transport, install, connect, and maintain their gas supplies, as well as to deal with emergencies. In the first of a two-part series, this month’s “GC Connections” examines the principal hazards and safety issues surrounding the compressed gas cylinder. Next month’s instalment will present safe procedures for routine cylinder use.
John V. Hinshaw, GC Connections Editor
Many gas chromatographers are not fully aware of safe practices for handling high-pressure gas cylinders. Gas chromatography (GC) operators should be trained to properly transport, install, connect, and maintain their gas supplies, as well as to deal with emergencies. In the first of a two-part series, this month’s “GC Connections” examines the principal hazards and safety issues surrounding the compressed gas cylinder. Next month’s instalment will present safe procedures for routine cylinder use.
The following is a concept script for a gas safety video. Readers are encouraged to find as many safety violations or bad practices as they can. Monday morning, 10:02 am, in a small chromatography lab. While starting up the gas chromatographs and lighting their flame detectors, Sam finds that one of the helium cylinders in the laboratory has gone empty over the weekend. He reaches over the other gas cylinders, applies a large tank wrench, and accompanied by a loud hissing sound, detaches the regulator fitting from the tank. Letting the regulator hang by its plastic connecting tubing, he moves the hydrogen and air cylinders out of the way into the space between the laboratory benches, tilts the empty cylinder on its bottom edge, and rolls it into position near the door.
Sam leaves the laboratory and returns in a moment pushing a furniture dolly. With a grunt, he tilts the cylinder sideways onto the dolly and, pushing it along, saunters down the corridor whistling the “Heigh-ho, Heigh-ho” theme from Disney’s 1950s Snow White. His coworker Amanda looks at him aghast as she heads into the laboratory.
Ten minutes later Sam returns with a new cylinder on the dolly. He lifts the tank up to a vertical position and the dolly rolls off, banging against the laboratory bench as Amanda jumps out of the way. Without bothering to strap any of the cylinders in place, Sam ducks down slightly and cracks open the new cylinder’s stem valve. Amanda is startled by a 110-decibel roar as the escaping gas expresses its new freedom.
Satisfied with the demonstration, Sam rolls the tank into position and reattaches the regulator. Then he starts to secure the other tanks. Amanda calls his name out loudly, “Sam, what do you think you’re doing?” As he spins around to deliver a clever reply, his belt buckle catches one of the dangling gas lines. In slow motion, the hydrogen tank starts to head for a horizontal position. Its valve and regulator glance off the bench top on the way down. The cylinder heads for the walls, and in a flash a bright orange-yellow light fills the laboratory . . .
Certainly, no one would take all of the wrong actions that this video dramatizes, but how many of us have done just one of them? I’ve witnessed them all, and I’m guilty of a few myself from time to time, especially in exceptional situations such as setting up a demonstration in a conference room. I sincerely hope that everyone in the laboratory treats flammable solvents and toxic chemicals with well-deserved respect and understands the short- and long-term hazards involved with handling hazardous materials. So, what leads some of us to fall short of giving compressed gas cylinders the respect they deserve? In terms of stored potential kinetic energy, they are bombs waiting to explode; in terms of suffocation potential or flammability, they can be just as much a fire hazard and as potentially toxic as any number of solvents and solids.
Periodically, “GC Connections” reviews gas cylinder safety. It’s been a while since the topic was last touched (1,2), so let’s take another look at the hazards gas cylinders present and some procedures and practices that can maximize safety for those who must work with them.
Cylinder Hazards
Gas cylinders present several obvious and some less-familiar hazards, including sudden decompression that can propel a cylinder remarkably quickly across the laboratory and displace breathing air; the risk of explosion or reaction; possible acute toxicity; heavyâobject hazards; and personal injury from highâpressure gas streams or cryogenic liquids. For reference, the Occupational Safety and Health Administration (OSHA) regulations 29CFR, Parts 1910.101–105 (3) provide specific guidelines for the use of compressed gases in the workplace that should be followed strictly. An excellent practical gas-safety document can be found on-line as well (4). These procedures and guidelines are discussed in more detail in the second part of this two-part series.
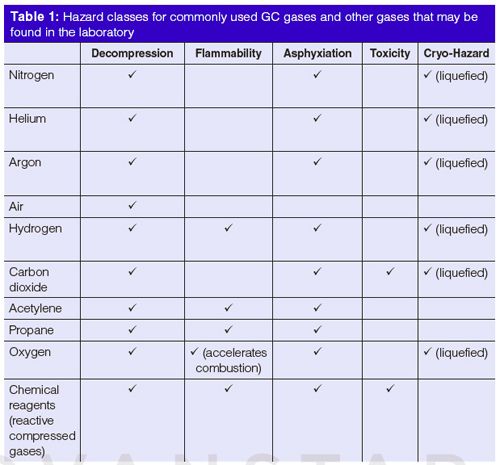
Table 1 lists hazard classes for commonly used gas chromatography (GC) gases. Gas chromatographers do not normally use some of the common hazardous gases in pure form such as acetylene, oxygen, nitrous oxide, or propane. These gases may be present in laboratories where other instrumentation is used, such as atomic absorption (AA) or atomic emission (AE) spectrometers. Everyone in the laboratory should be aware of the extra dangers posed by chemically reactive, fuel, or oxidizer gases.
Decompression
The first thought that comes to mind when discussing gas cylinders is their rocket potential. A 1-A size cylinder of helium contains 8.3 m3 (293 ft3) of room-pressure gas that’s compressed into a space of less than 0.5 m3 (2.0 ft3) at a nominal fill pressure of 18.1 MPa (2640 psi). European “L” size cylinders contain slightly more compressed gas. These cylinders weigh approximately 91 kg (200 lb) when empty, and the weight of helium contained in a fully pressurized cylinder is around 1.4 kg (3 lb). When the gas pressure is released rapidly through an opening the size of the valve stem, the cylinder - if it accelerates in a straight line - can reach velocities of close to 30 m/s, 108 km/h, or 66 mph. A 91-kg metal cylinder hurtling at high velocity can do tremendous damage almost instantaneously, and there is nothing that a person can do to stop it once a decompression incident starts. See the sidebar “How Fast Will a Cylinder Go” for the calculations that produced this velocity figure.
The thought of a heavy cylinder careening through the laboratory walls gets the attention of most lab workers. This type of accident is easy to avoid by always restraining cylinders with appropriate chains or brackets, transporting them in cylinder carts, and keeping them capped at all times unless actually in use with a regulator or manifold attached. Any cylinder that is found to be damaged or has a stuck valve should be returned immediately to the supplier. If the damage is to the cylinder body the supplier should be notified to come and remove it. Never try to vent a damaged cylinder.
Asphyxiation
Even though the cylinder is restrained, another problem can occur when the contents of any large gas cylinder - other than an air cylinder - are vented rapidly. The sudden release of over 8 m3 of unbreathable gas in the laboratory may reduce the level of oxygen in the air drastically and present a real suffocation hazard. Liquefied gases expand by as much as 1000-fold when vaporized and can present a much greater hazard. Liquid nitrogen Dewars contain enough nitrogen gas to make a room incapable of sustaining life if the gas is released rapidly. Carbon dioxide can cause immediate unconsciousness followed by death when breathed in any significant concentration. It is much denser than air and will settle in low unventilated areas. Liquid carbon dioxide tanks, such as used for GC oven cooling, can release especially large quantities of gas during a tank rupture.
If an event such as this happens, leave the area immediately, prevent others from entering the laboratory, and seek the assistance of personnel trained in the use of a self-contained breathing apparatus. Without the proper breathing equipment, never try to re-enter a hazardous area to assist someone else. Some companies have such equipment on-site, but many rely upon emergency services to enter the affected area. Always make sure the area has been well ventilated before returning. Many unnecessary tragedies have occurred due to misguided rescue attempts.
Explosion and Fire Hazards
If a hydrogen cylinder vents into the laboratory in an uncontrolled manner, even if the leak is through the pressure-release disc on the cylinder or regulator, leave the area immediately, close the doors, pull the fire alarm to evacuate the building, and call emergency services. Don’t try to extinguish flame detectors, or shut down anything else in the laboratory - just get out quickly. Hydrogen has a lower explosive limit (LEL) in air of 4%, so a venting cylinder can easily create an explosive concentration in moments. In its favour, hydrogen rapidly diffuses in air so that venting the flows encountered in flame detectors or when used for carrier gas present no significant hazard under normal conditions. However, hydrogen can accumulate in a closed GC oven in the event of a broken column. Most electronic pressure control (EPC) systems incorporate flowâmonitoring safety features that will detect this condition and shut down the carrier-gas flow.
The same evacuation procedure is required with other flammable gases like propane and acetylene or reactive gases and oxidizers such as oxygen and nitrous oxide. Breathing air contains about 20% oxygen, but high oxidizer concentrations will accelerate combustion dangerously and can cause serious burn injuries. Remember that clothing, paper, paint, and plastic can all burn rapidly in the presence of high oxidizer concentrations.
If a gas fire starts and the gas leak cannot be stopped safely and positively, don’t try to extinguish the flame. Unburned gas may accumulate and explode if an ignition source is present.
Hydrogen particularly presents a special hazard because it burns in air with an invisible flame. Never try to investigate a possible hydrogen fire by approaching the suspected flame area: leave it to the professionals. Although the combustion byproducts of hydrogen are nontoxic, the fire may burn other nearby items such as plastics, which can produce toxic combustion byproducts.
High-pressure gas cylinders can rupture explosively when heated in a fire. All cylinders include a thermal fuse that is supposed to melt and release the cylinder contents in a semicontrolled manner before the internal pressure exceeds a safe upper limit. However, if the cylinder has been mechanically stressed by falling over or from the impact of another cylinder, it can burst before the pressure release valve can act. A chain-reaction effect sometimes occurs in large fires in areas where many cylinders are stored.Toxicity
GC gases generally aren’t toxic. That is, after a victim has been removed from an accident area and has received first aid the immediate effects of inert gas exposure, such as dizziness and difficulty breathing, will rapidly diminish. Chemically active sample or reaction gases, on the other hand, can present a real toxic health hazard and a significant disposal problem. If even a small leak of a toxic gas such as carbon monoxide or ammonia is detected, leave the area and call in trained personnel to remove the leaking cylinder to a safe place.
Each type of gas or gas blend has an associated material safety data sheet (MSDS) that must be sent in advance to the purchaser who must then keep the information on file for access by any employee or emergency response personnel. MSDSs contain extensive information about the use, storage, and disposal of chemicals - including compressed gases - their toxicity, and any other relevant information. Refer to the appropriate MSDS when you have any questions about a particular material.
Many years ago I saw lecture bottles of methyl bromide, hydrogen fluoride, carbon monoxide, and various highly reactive silanes - not all in the same laboratory, fortunately - carelessly stored on shelves above floor level with unprotected valves. No analytical or chemical laboratory can justify operation with such hazards present. Improperly stored or deployed toxic gas cylinders have no place in anyone’s workplace. If any are found, it’s good procedure to evacuate the area and call in a hazardous materials team to remove the danger. In any case, never try to move or dispose of hazardous or unknown chemicals in gaseous, liquid, or solid form yourself - it’s not worth the risk.
Heavy Lifting
No one should try to lift a cylinder that weighs more than about 12 kg (26 lb). Heavy cylinders belong on the floor, restrained to a bench or a wall. Always use a cylinder cart to move cylinders around, even from one part of the laboratory to another. The practice of rolling a cylinder on its bottom edge, while prevalent, risks injury to feet - and the risk of the cylinder becoming unbalanced and falling over. Never place a cylinder on its side and roll it: the sidewalls are the thinnest parts and aren’t designed to take any weight. You could be creating a dangerously weak cylinder that may explode the next time it’s filled with gas.
Liquid carbon dioxide cylinders, used for cryogenically cooling GC ovens, weigh much more when full because of liquid carbon dioxide’s density, and they can be deceptively heavy. Always pay special attention to these cylinders. In all cases, it’s good practice to wear protective eyewear, shoes, gloves, and clothing when manipulating large gas cylinders.

Cryocooling
Cryogenic liquefied gases such as liquid nitrogen or carbon dioxide present additional hazards in the laboratory. Carbon dioxide, a liquid when stored under pressure at room temperature, cools to subzero temperatures when decompressed because of both expansive and evaporative cooling. Liquid nitrogen is stored under low positive pressure in special Dewar tanks at around -195 °C. Both cryogenic gases can cause immediate frost burns on exposed skin. Liquid nitrogen also presents a cryogenic freezing hazard that embrittles almost any object it contacts in bulk, including fingers. Connecting tubing that conducts cryogenic liquids also presents a freezing hazard - the tubing should always be insulated or shielded to prevent accidental contact. Again, appropriate protective measures such as thermal gloves, eyewear, and skin-covering clothing help prevent accidents.
High Pressure
The hapless lab rat in the video liked to crack open the high-pressure valve with no regulator attached. I suppose the idea is to blow out any dust particles as well as to see if the tank is pressurized, but this behaviour is never a good idea. The force exerted by gas decompressing from high pressures is tremendous. If he happened to have part of his hand or arm in front of the cylinder fitting he could suffer a serious abrasion, deep cut, or worse. A much better way to clear the dust out is to spray the area with clean, dry compressed air from a good air source. Never spray a halocarbon-based material onto the cylinder fitting - the gas can get into the lines and cause problems with electron-capture and mass spectrometry detectors.

Conclusion
I’ve addressed many of the hazards associated with compressed and liquefied gases in this month’s “GC Connections”. The four most important considerations when dealing with compressed gas cylinders are proper physical restraint, personal protection, knowledge of potential hazards, and appropriate emergency procedures. After a cylinder is in place in the laboratory, the next step is to hook it up and put it in service. In the next instalment I’ll present some good procedures to follow when installing, using, and replacing gas cylinders and pressure regulators.
References
- C. Hallenbeck and D.F. Gill, LCGC North Am.25(1), 40–47 (2007).
- J.V. Hinshaw, LCGC Europe27(3), 144–148 (2014).
- Code of Federal Regulations (CFR), 29 CFR 1910.101, “Compressed Gases” (U.S. Government Printing Office, Washington, D.C., USA). Available at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9747.
- “Compressed Gas Safety Guide,” at http://www.stonybrook.edu/facilities/ehs/occupational/cg.shtml (SUNY Stony Brook University, USA, September, 2016).
“GC Connections” editor John V. Hinshaw is a senior scientist at Serveron Corporation in Beaverton, Oregon, USA, and a member of LCGC Europe’s editorial advisory board. Direct correspondence about this column should be addressed to “GC Connections”, LCGC Europe, Hinderton Point, Lloyd Drive, Ellesmere Port, Cheshire, CH65 9HQ, UK, or e-mail the editor-inâchief, Alasdair Matheson, at alasdair.matheson@ubm.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











