Gas Chromatography–Vacuum Ultraviolet Absorbance Spectroscopy for the Quantitative Determination of Trace and Bulk Water in Organic Solvents
Recent advances in vacuum ultraviolet (VUV) spectroscopy have allowed for the application of this technology as a chemical detection platform for gas chromatography (GC). This technique is known as GC–VUV. A GC–VUV detector can produce highly characteristic absorbance spectra for nearly all chemical species in the wavelength region of 125–240 nm. This enables not only identification but also robust quantitation of a variety of compounds separable by gas chromatography, including water. This article describes the results of a pilot study focused on trace water determination in common organic solvents using an ionic liquid stationary phase GC column in a GC–VUV platform.
Photo Credit: Yuri Samsonov/Shutterstock.com

Lindsey Shear-Laude, VUV Analytics, Cedar Park, Texas, USA
Recent advances in vacuum ultraviolet (VUV) spectroscopy have allowed for the application of this technology as a chemical detection platform for gas chromatography (GC). This technique is known as GC–VUV. A GC–VUV detector can produce highly characteristic absorbance spectra for nearly all chemical species in the wavelength region of 125–240 nm. This enables not only identification but also robust quantitation of a variety of compounds separable by gas chromatography, including water. This article describes the results of a pilot study focused on trace water determination in common organic solvents using an ionic liquid stationary phase GC column in a
GC–VUV platform.
The determination of water content is a required measurement in many sectors of chemical industry including pharmaceutical, specialty chemical, petrochemical, semiconductor, food and beverage, and environmental monitoring. It is currently one of the most common and important routine measurements made in the chemical industry. Developing accurate, versatile, and efficient analytical methods for the quantitative determination of water in a variety of matrices is a relevant and challenging analytical problem. Water is inherently difficult to measure because of its ubiquitous presence. Several different approaches to water determination have been reported in the literature including gravimetric techniques, Karl Fischer Titration (KFT), gas chromatography (GC), nearâinfrared spectroscopy (NIR), solvatochromic sensing, fluorine nuclear magnetic resonance spectroscopy (FNMR), and isotope ratio mass spectrometry (IRMS) (1–3).
Currently Karl Fischer titration, which was first reported in 1935, is the most widely used analytical technique for the quantitative determination of water; however, it has many limitations. KFT is based on a series of chemical reactions which require relatively large volumes of hazardous reagents and sample. These reagents can be subject to side reactions as well as exhibit long-term stability issues because of chemical reactivity and because they are hygroscopic in nature. Although there are many different variations on basic KFT methods (for example, coulometric KFT) that have been developed to minimize these issues, questions still remain regarding degradation of reagents over time and residual water present in KFT reagents. The fact remains that, although KFT is the most widely used technique for water determination, interfering side reactions, reagent instability, sample insolubility, pH issues, and the complexity of the analysis has prevented KFT from becoming a universal method for this purpose. Coulometric KFT is also prone to electrochemical drift and environmental ingress during the measurement, which often leads to poor accuracy and precision unless adequate standardization protocols are adhered to. This can lead to significant consumption of valuable time and reagents. Because of these challenges, the analytical utility of Karl Fischer titration also suffers at analyte concentrations less than 1000 ppm H2O with the most pronounced difficulties being present at analyte concentrations less than 100 ppm H2O.
Gas chromatography–vacuum ultraviolet spectroscopy (GC–VUV) offers a powerful, yet simple alternative for the determination of water in organic solvents, while also allowing for unique spectral identification of other chemical components in the sample via the measurement of characteristic VUV absorbance spectra. The VUV detector provides rapid measurement of absorbance spectra from 125–240 nm, where nearly all chemical species absorb, making it a nearly universal detection platform for GC. GC–VUV has been shown to be a powerful addition to the analytical toolbox in the measurement of hydrocarbons, fixed gases, polycyclic aromatic hydrocarbons, fatty acids, multiclass pesticides, drugs, estrogens, fatty acid methyl esters (FAMES), volatile organic contaminants (VOCs), and other volatile compounds (4–9).
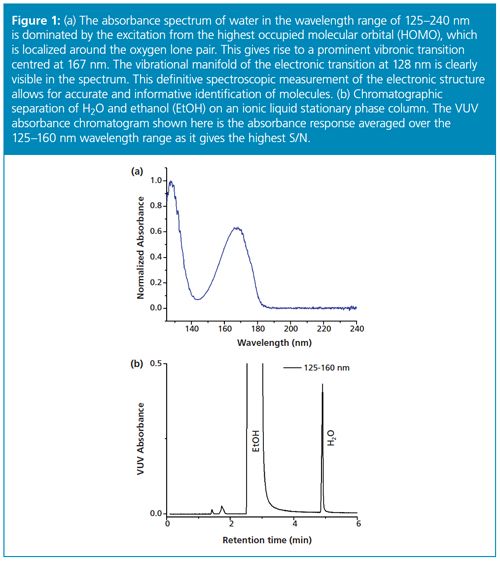
The VUV detector is also sensitive and selective for water, and achieves detection limits lower than those achieved with conventional KFT techniques operated at ambient laboratory conditions. Before the advent of this technology, water analysis using gas chromatography had been possible using GC–TCD (thermal conductivity detection). However, this approach does not give characteristic chemical information. GC–VUV offers a robust, sensitive, and selective approach for quantitative water determination with the advantages of modern analytical chromatography. The VUV absorbance spectrum for water is shown in Figure 1(a) and gives rise to a prominent vibronic transition centred at 167 nm. The vibrational manifold of the electronic transition at 128 nm is also clearly visible in the spectrum. This definitive spectroscopic measurement of the electronic structure of water in the gas phase allows for accurate and informative identification of water using VUV spectral library matching.
One pervasive challenge to the measurement of water on GC systems has been the lack of columns suitable for chromatographic analysis of water. With the recent advent of ionic liquid stationary phase GC columns, water analysis by GC has become a promising analytical solution. The aim of this study is to demonstrate the capabilities of an ionic liquid–based capillary GC–VUV method for quantitative water analysis in a variety of solvents and to demonstrate the analytical figures of merit for this technique.
Experimental
An Agilent 6890 gas chromatograph was coupled to a VGA-100 VUV absorbance detector (VUV Analytics, Inc.) and used to measure the amounts of water present in several organic solvents. The data collection rate of the detector was set to 4.5 Hz. The transfer line and flow cell temperatures of the detector were set to 275 oC, and the make-up gas (nitrogen) was set to 0.250 psi. A 30 m × 0.25 mm, 0.20-µm Watercol 1900 column (Supelco) was used and was operated in constant flow mode (3 mL/min) with helium carrier gas. The GC oven profile was set to start at 40 oC (no hold) and then increased to 180 oC at a rate of 10 oC/min. The inlet was operated with a split ratio of 5:1. The inlet temperature was set to 250 oC. All injections were performed using a 7683 Series Auto injector (Agilent Technologies). All solvents used in this investigation were purchased from Sigma Aldrich and were HPLC grade. The 3-Å molecular sieves and Hydranal WaterâinâMethanol 5.0 standard were purchased from Sigma Aldrich as well.
All glassware used during this analysis was oven dried at 250 oC for 24 h and stored in a desiccator before use. All solvents analyzed were dried by storing over 25% w/w 3-Å molecular sieves in a desiccating environment for a minimum of 24 h. All solvents were then transferred to dried GC vials and GC–VUV analysis was performed. A method of standard additions (MSA) approach was used to determine the residual level of water present in multiple organic solvents upon drying and to validate the accuracy of the method.
Results and Discussion
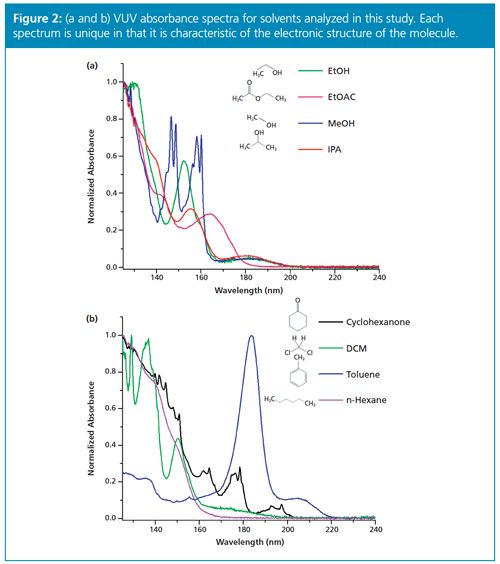
The method showed excellent chromatographic resolution and peak shape for water as well as the characteristic determination via the measured VUV absorbance spectrum. An example chromatogram showing water in ethanol (EtOH) can be seen in Figure 1(b). The VUV absorbance spectrum of water is also shown in Figure 1(a). The method is simple, quick, and efficient and does not require the use of chemical reagents. Several organic solvents were analyzed in this study including ethanol, methanol, isopropyl alcohol, acetonitrile, ethyl acetate, cyclohexanone, dichloromethane, toluene, and n-hexane. The VUV absorbance spectra measured for each of these solvents is shown in Figure 2. The method yielded robust water separation for each solvent analyzed in this study.
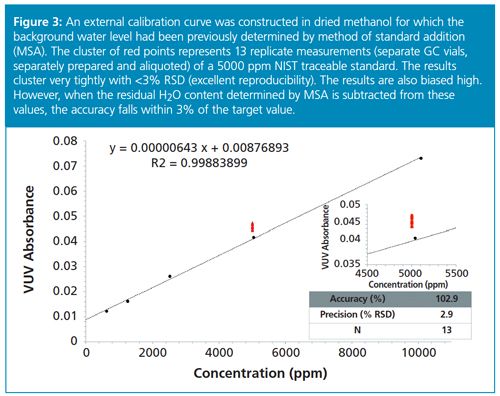
GC–VUV exhibits a dynamic linear range for water between 10 ppm and 10,000 ppm (Figure 3). The linear response for standard addition curves for water in dried solvents allows for the accurate determination of residual water content. Once the background level of water has been accurately determined, this level can be subtracted from unknown values determined with subsequent calibrations. Residual water is a result of moisture present at ambient laboratory conditions, the types of surfaces contacted by the solvent, and the relative polarity of the solvent, as well as the degree of water removal via molecular sieves. Accuracy and precision were determined for 13 replicate measurements of Hydranal-Water in Methanol 5.0 (a 5000 ppm NIST traceable standard). Accuracy and precision were both less than 3.0%, thereby indicating a high level of metrological quality for the GC–VUV measurement of moisture in methanol. Similar results have been obtained for water in a variety of organic solvents (10).
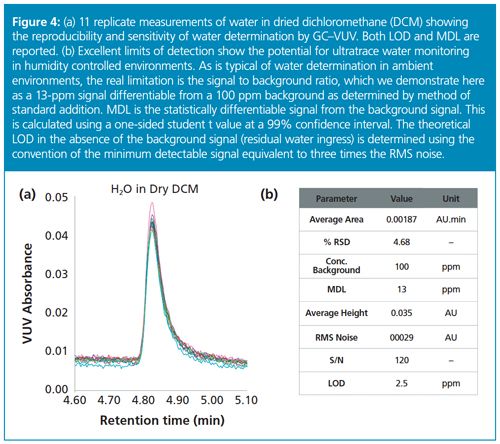
Reproducibility and detection limits were determined for this method using dry dichloromethane (DCM) as shown in Figure 4. Replicate measurements of residual water content showed good precision and low limits of detection. This analysis offers excellent results compared with the conventional methods that have been discussed. One key advantage of using GC–VUV to determine water is that relatively small changes in water content (<20 ppm) are readily measureable above residual concentrations because of the reproducibility of measurements and low signal-to-noise.
Conclusions
The initial results from this pilot study show that gas chromatography coupled with vacuum ultraviolet absorbance spectroscopy is a powerful tool for the quantification of trace water in organic solvents. These preliminary studies show that ionic liquid stationary phase GC columns are suitable for rapid and robust separations of trace amounts of water from numerous organic solvents. VUV detection is sensitive, accurate, precise, and can be used to determine water content in samples at levels less than 100 ppm under ambient conditions. Future applications of this technology to petrochemical, pharmaceutical, and quality assurance are shown to be feasible using a validated method based on the technique demonstrated here.
Acknowledgements
The author gratefully acknowledges the contributions from Len Sidisky at Supelco.
References
- J.M. Hogan, R.A. Engel, and H.F. Stevenson, Anal. Chem. 42, 249–252 (1970).
- W.K. O’Keefe, F.T.T. Ng, and G.L. Rempel, J. Chromatogr. A.1182, 113–118 (2008).
- G.M.T. de Jong, J.R. Huizenga, B.G. Wolthers, H.G. Jansen, D.R.A. Uges, F.R. Hindriks, and C.H. Gips, Clin. Chim. Acta.166, 187–194 (1987).
- T. Gröger, B. Gruber, D. Harrison, M. SarajiâBozorgzad, M. Mthembu, A. Sutherland, and R. Zimmermann, Anal. Chem.88, 3031–3039 (2016).
- K.A. Schug, I. Sawicki, D.D. Carlton, H. Fan, H.M. McNair, J.P. Nimmo, P. Kroll, J. Smuts, P. Walsh, and D. Harrison, Anal. Chem. 86, 8329–8335 (2014).
- L. Bai, J. Smuts, P. Walsh, H. Fan, Z. Hildenbrand, D. Wong, D. Wetz, and K.A. Schug, J. Chromatogr. A. 1388, 244–250 (2015).
- C.A. Weatherly, Y. Zhang, J.P. Smuts, H. Fan, C. Xu, K.A. Schug, J.C. Lang, and D.W. Armstrong, J. Agric. Food Chem.64, 1422–1432 (2016).
- H. Fan, J. Smuts, L. Bai, P. Walsh, D.W. Armstrong, and K.A. Schug, Food Chem.194, 265–271 (2016).
- H. Fan, J. Smuts, P. Walsh, D. Harrison, and K.A. Schug, J. Chromatogr. A.1389, 120–127 (2015).
- L.Shear, L. Sidisky, and D. Harrison, “Quantitative Water Determination by Gas Chromatography-Vacuum Ultraviolet Absorbance Spectroscopy (GC-VUV),” poster #: 790-14P presented at Pittcon, Atlanta, Georgia, USA, 2016.
Lindsey Shear-Laude is an applications scientist at VUV Analytics Inc. She earned a B.S. in chemistry from the University of Texas at Austin and a MA in analytical chemistry from the University of Arizona. Her current interests are focused on advanced detector technology for gas chromatography.
E-mail: Lindsey.Shear@vuvanalytics.com
Website: www.vuvanalytics.com

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









