Gas Chromatography Tandem Mass Spectrometry Analysis of Ethylene Oxide: An Emerged Contaminant in Seeds and Spices
The occurrence of the banned insecticide, ethylene oxide (EtO), and its transformation product, 2-chloroethanol (2-CE), has recently been reported in various food commodities. In this study, two alternative approaches based on gas chromatography coupled to tandem mass spectrometry (GC–MS/MS) were developed to control maximum residue limit (defined as the sum of ethylene oxide and 2-chloroethanol expressed as ethylene oxide). The first approach offered a rapid screening of 2-CE (the contamination marker) in an aqueous acetonitrile extract purified by dispersive solid-phase extraction (dSPE). The total EtO was determined by the second approach, which involved conversion of EtO to 2-CE by acid hydrolysis in the presence of chloride; the ethyl acetate extract was purified prior to instrumental analysis by dSPE. The achieved limit of quantification for EtO (the sum of EtO and 2-CE expressed as EtO) was low enough to ensure compliance with regulatory requirements. The accuracy of the results was successfully verified by analysis of the EUPT test material (EUPT-SRM16 - 2021).
Ethylene oxide (EtO) is a gaseous disinfectant that was, due to its toxicity, banned in the EU as a pesticide in 1991 and as an output product in food and feed in 2011. It is classified as a category 1 carcinogen by the International Agency Research of Cancer (IARC). In September 2020, Belgium reported via the Rapid Alert System for Food and Feed (RASFF) an occurrence of EtO in sesame seeds. The concentration of this active ingredient of insecticidal fumigants exceeded the maximum residue level (MRL) of 0.05 mg/kg set for this commodity in the Regulation (EC) NO 396/2005 (1) by six hundred times (the residue definition involves not only EtO but also its transformation product, 2-chloroethanol [2-CE], expressed as EtO). Up to September 2021, 629 notifications in RASFF related to the occurrence of EtO, mostly in seeds (particularly sesame seeds) and spices, have been registered. Contamination of various food additives, (for example, E410 and E416) and various food items, such as ice creams and desserts, milk products, pastries, breads, and a number of other products containing ingredients illegally processed with EtO, was also reported.
Due to a high reactivity, EtO used to be applied in the sterilization of health care material surfaces and for the treatment of spices and seeds in some countries (2–4). If the aeration step needed for spices and seeds is not properly performed, residues of EtO may spontaneously react with chloride ions commonly present in a particular matrix. In this way, 2-CE originates during storage, and thus becomes a suitable marker of the use of EtO for fumigation.
Until now, a laboratory-performed EtO control in foodstuffs has not been routinely performed, and only a few methods describing an analytical procedure have been published. Unfortunately, most methods are based on time-consuming sample preparation steps, including conversion of EtO to 2-CE using sodium iodide prior to analysis (5,6).
This study presents two simple validated gas chromatography tandem mass spectrometry (GC–MS/MS) methods, the first being dedicated to a rapid screening of 2-CE (the marker of EtO-based fumigation) in sesame seeds and spices, the second one to enable determination of total EtO concentration, in line with the regulatory requirements (MRL control).
Materials and Methods
Chemicals: Standards of EtO and 2-CE were purchased from Sigma Aldrich. 2-chloro-1,1,2,2-tetradeuterioethanol (D4-2-CE), an internal standard, was purchased from HPC Standards GmbH. Analytical‑grade ethyl acetate and acetonitrile were obtained from Merck. A Milli-Q Integral System supplied by Merck was used throughout the study. Magnesium sulphate and a Z-Sep+ sorbent were delivered from Sigma Aldrich. C18 and PSA sorbents were supplied by Agilent Technologies. Sodium chloride and sulphuric acid (96%) were purchased from Lach-ner and Penta, respectively.
Two sets of calibration solutions with 2-CE concentration 2, 12, 20, 50, 100, 200, 500, 1000, and 2400 ng/mL were prepared in ethyl acetate and in acetonitrile. Each calibration level contained 100 ng/mL of D4-2-CE.
To test EtO conversion to 2-CE, an EtO standard solution of 5000 ng/mL methanol was prepared.
Samples: Spice samples (ground pepper and paprika) and hulled bio sesame seeds obtained at a retail market were used for procedure optimization and validation experiments. For the quality control (QC) test material, the proficiency testing sample (sesame seeds) obtained from EUPT-SRM16 - 2021 was used (7).
Sample Preparation:
Solid-Phase Microextraction: A 1 g measure of the sesame seeds sample was mixed with 2 mL of 0.2 M H2SO4 and 0.5 mL of a saturated solution of NaCl. The samples were spiked by 2-CE at different concentration levels from 0.006 to 0.6 mg/kg and analyzed by solid-phase microextraction and gas chromatography coupled to high resolution mass spectrometry (SPME–GC–HRMS).
Strategy No. 1: Extraction/Purification: A 2 g amount of the representative sample (D4-2-CE used as a surrogate was added at concentration 0.5 mg/kg) was mixed with 10 mL (v/v) 95:5 acetonitrile–water in a polypropylene centrifuge tube and shaken for 30 min. The tube was then centrifuged (5 min, 10,000 rpm, Rotina 35R, Hettich Zentrifugen). A 3 mL measure of supernatant were transferred into the polypropylene centrifuge tube and 0.2 g of Z-Sep+ and 0.2 g of MgSO4 were added. The mixture was shaken for 2 min and centrifuged (3 min, 10,000 rpm). Finally, the extract was transferred into vials for GC–MS/MS analysis.
Strategy No. 2: Extraction/Purification: A 2 g amount of the representative sample (D4-2-CE used as a surrogate was added at concentration 0.5 mg/kg) was mixed with 5 mL 0.2 M H2SO4 and 1 mL of a saturated solution of NaCl in a polypropylene centrifuge tube. After 2 h of sonication at 50 °C, 10 mL of ethyl acetate was added and the mixture was shaken for 15 min and then centrifuged (5 min, 10,000 rpm). An aliquot of 3 mL was removed from the upper organic layer and 0.075 g of PSA, 0.075 g of C18, and 0.2 g of MgSO4 were added. The mixture was shaken for 2 min and centrifuged (3 min, 10,000 rpm). Finally, the extract was transferred into vials for GC–MS/MS analysis.
To test the yield of EtO conversion to 2-CE during acid hydrolysis, a sample of sesame seeds was spiked at a concentration of 0.05 mg/kg and analyzed using the above mentioned procedure.
During the sample preparation process, blanks were also prepared consisting of all the steps except the addition of the sample.
Analyte Separation and Detection:
SPME–GC–HRMS: For a headspace solid-phase microextraction (HS–SPME), a 75 µm Carboxen/polydimethylsiloxane (CAR/PDMS) fibre obtained by Supelco was used. The optimized HS-SPME extraction conditions were: incubation time, 120 min; incubation temperature, 50 °C; agitator speed, 500 rpm; extraction time, 20 min; desorption temperature, 250 °C; desorption time, 2 min; and the analytes were extracted in headspace. EtO analysis by SPME was performed using a GC–MS system consisting of a 7890A gas chromatograph (Agilent Technologies) equipped with a multifunctional autosampler MPS 2 (Gerstel) and coupled to Pegasus HRT ultrahigh-resolution time-of-flight mass spectrometer (Leco). For the separation, a 50 m × 0.20 mm, 0.20-µm HP-INNOWax capillary column (column no. 1) (Agilent Technologies) was used. A sample was injected in split mode (1:5). The oven temperature programme was as follows: 35 °C (8 min), 50 °C/min to 220 °C (hold 10 min). The temperatures of the transfer line and ion source were 220 and 230 °C, respectively. The mass spectrometric detector was operated in the electron ionization mode. The mass range was 35–550 m/z and the resolution of the mass analyzer was set > 25,000 (FWHM).
Strategy No. 1 and 2: GC–MS/MS: A GC–MS/MS system consisting of an Agilent 7890A gas chromatograph equipped with multimode inlet and triple quadrupole mass spectrometer Agilent 7000b MS operated in EI mode was employed for the analysis of 2-CE.
The separation of EtO and 2-CE was performed on a 30 m × 0.2 mm, 1.12‑µm HP-VOC capillary column (column no. 2) (Agilent Technologies) using the oven temperature program 45 °C (2 min), 20 °C/min to 240 °C (5 min). A 2 µL sample was injected in splitless and split mode (1:4) at 250 °C.
For the analysis of 2-CE after both types of extraction techniques, a 30 m × 0.25 mm, 0.25-µm HP-INNOWax capillary column (column no. 3) (Agilent Technologies) was used. A 2 µL volume was injected in splitless mode at 250 °C. The oven temperature programme was as follows: 57 °C (1 min), 20 °C/min to 130 °C, 5 °C/min to 140 °C (post run at 230 °C for 25 min).
Triple quadrupole was operated in multiple reaction monitoring (MRM) mode detecting transitions for EtO (44"29, 44"28, 44"19), 2-CE (80"31, 82"31, 80"43), and D4-2-CE (84"34, 86"47, 86"34, 84"47) at collision energy 5 eV. The temperatures of the transfer line, ion source, and 1st and 2nd quadrupole were 220, 280, 150 and 150 °C, respectively. The collision cell gases were nitrogen (1.5 mL/min) and helium (2.25 mL/min).
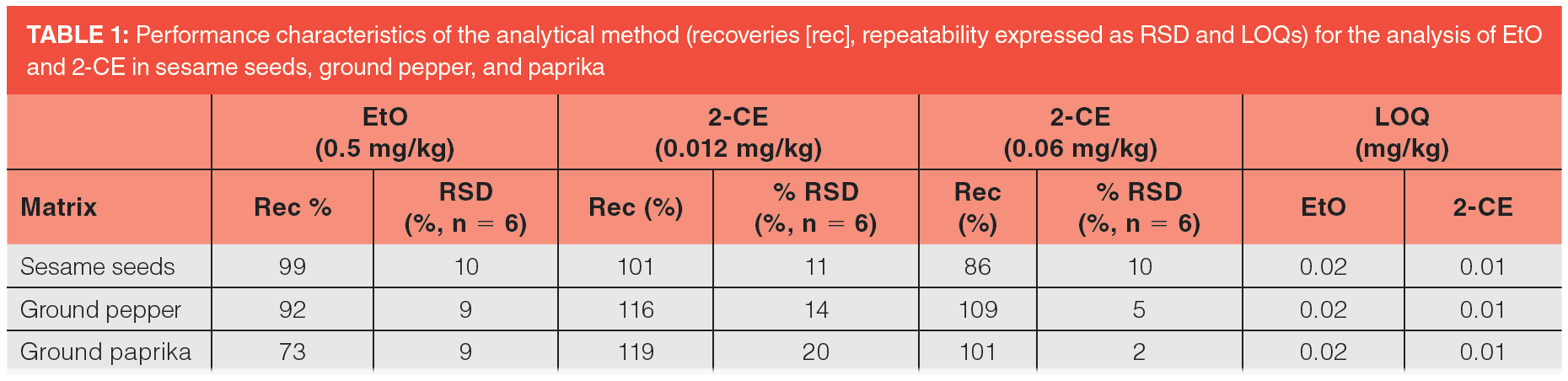
Validation: The method validation (strategy no.1 and no.2) was performed by analyzing ground pepper, ground paprika, and hulled sesame seeds (each matrix was analyzed in six replicates). To validate the method for the determination of 2-CE by acetonitrile extraction, the samples were spiked with 2-CE at concentration levels 0.012 and 0.06 mg/kg, respectively. The method, including acid hydrolysis followed by ethyl acetate extraction for the analysis of EtO (conversion to 2-CE), was validated at the concentration level 0.05 mg/kg.
To verify a yield of EtO to 2-CE conversion, the sample of sesame seeds was spiked by EtO at concentration level 0.05 mg/kg in six replicates and the recovery was subsequently calculated.
Accuracy of the newly developed GC–MS/MS method was tested using external QC material (sesame seeds EUPT-SRM16 - 2021) analyzed by strategy no. 1 and no. 2.
Results and Discussion
As mentioned at the start of this article, the EtO MRL definition (the Regulation [EC] NO 396/2005) involves free EtO and EtO converted into 2-CE. While EtO is a reactive compound with a low boiling point (10.4 °C), the value for 2-CE is more than ten times higher (129 °C). To the best of our knowledge, both EtO forms may occur in tested samples; nevertheless, 2-CE dominates. It is worth noting that simultaneous determination of these two analytes, EtO and 2-CE, by GC is a difficult task due to fairly differing physicochemical and thus chromatographic properties. In our preliminary experiments, SPME was used for analyte headspace sampling followed by GC separation and MS detection (a high-resolution TOF mass analyzer was used for screening purposes). In the case of EtO, critical matrix interference occurred under testing conditions; the target analyte coeluted with acetaldehyde, a compound with the same elemental composition (C2H4O), similar Kovats retention index (RI) (EtO: 680, acetaldehyde: 690, both related to polyethylene glycol stationary phase), and a very similar mass spectrum. Moreover, in spite of the advanced instrumental technique used, the limit of quantification (LOQ, detectability of confirmation ion 80.0023 required) of 2-CE (0.05 mg/kg) was not low enough due to relatively high “chemical noise” (see Figure 1).
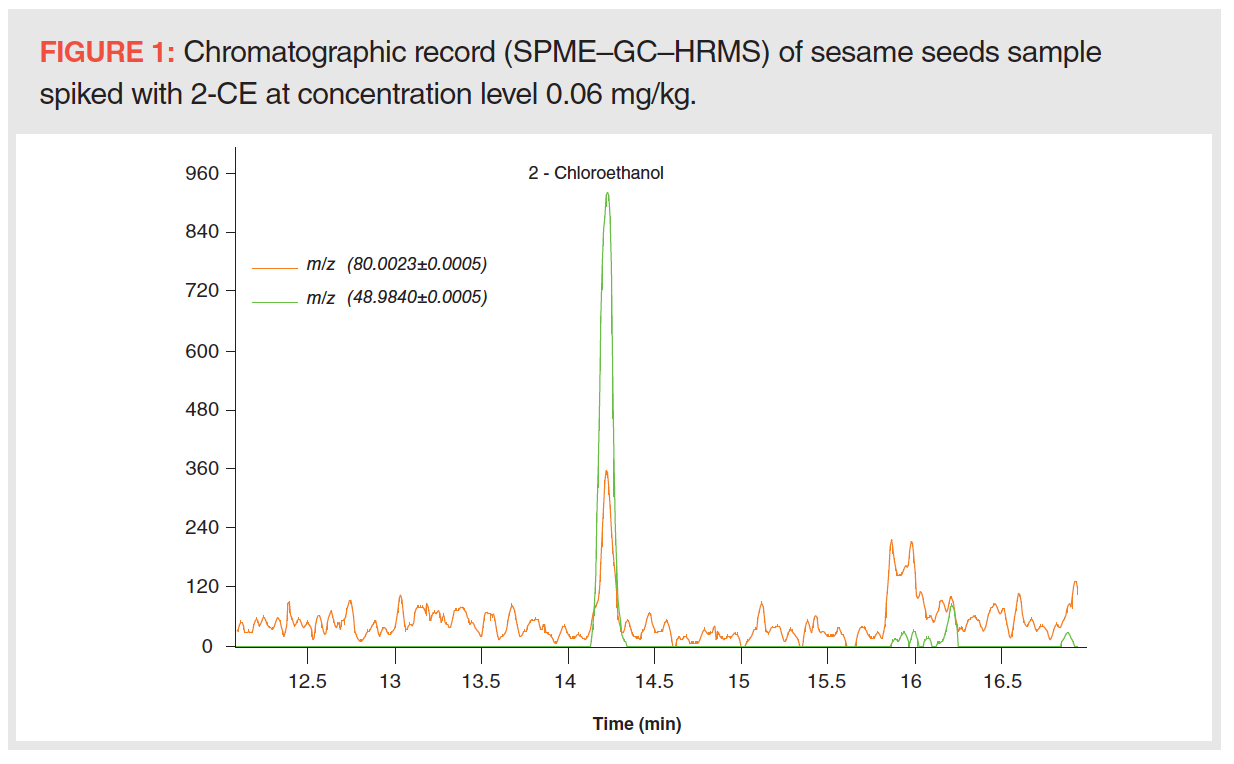
To overcome the issue of EtO and acetaldehyde coelution and to achieve a lower LOQ, column no. 2, with a thick film of stationary phase suited for the analysis of compounds with low boiling points, was used and an acetonitrile–water extract was injected into the GC–MS/MS system in split injection mode. Acetaldehyde and EtO were successfully separated; however, the lowest LOQs for EtO and 2-CE (see Figure 2) were only 0.5 and 0.2 mg/kg, respectively, and so did not enable MRL control (sesame seeds, MRL = 0.05 mg/kg for EtO – sum of EtO and 2-CE expressed as EtO; spices, MRL = 0.1 mg/kg for EtO – sum of EtO and 2-CE expressed as EtO). For lower LOQs, the splitless injection mode was also tested, but this injection technique negatively affected a peak shape of EtO and its detectability.
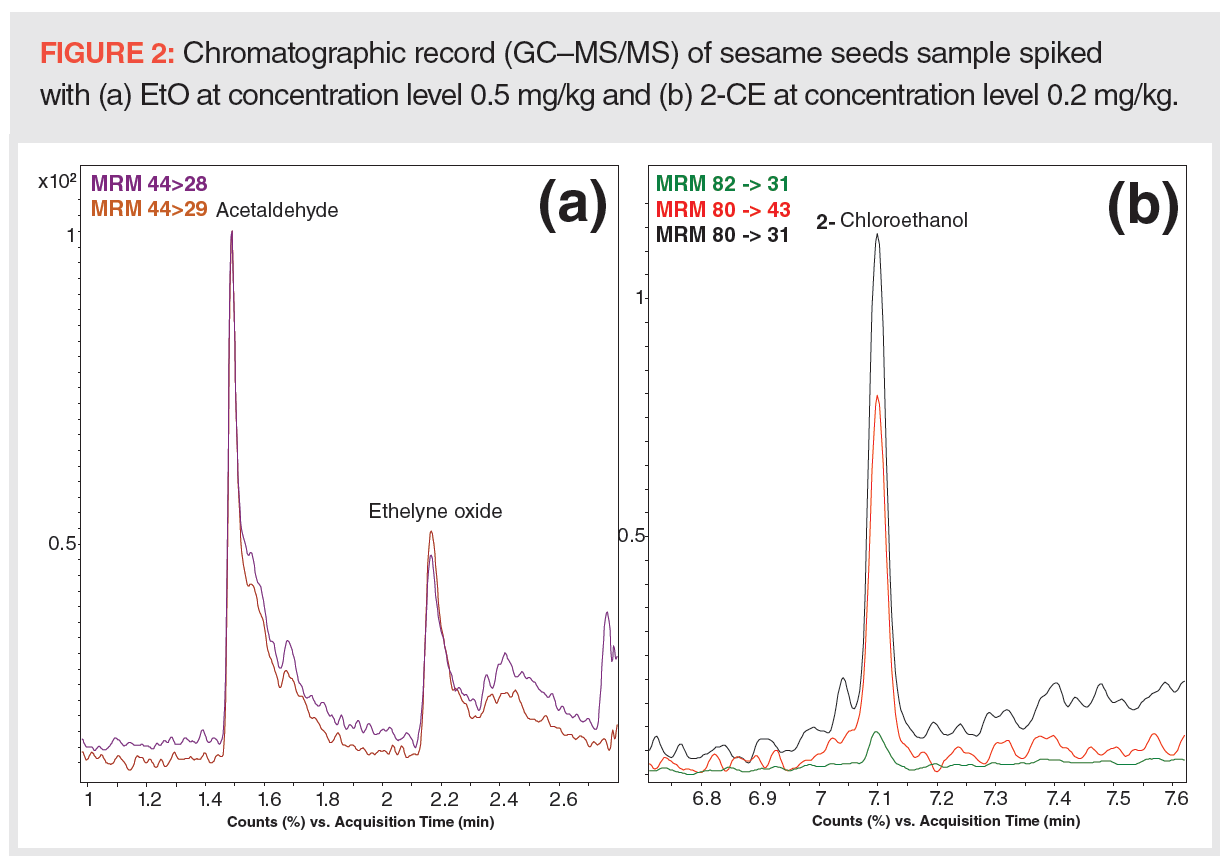
As the simultaneous GC–MS analysis of EtO and 2-CE was found to not be easily feasible, in the next phase, we focused on (i) screening of 2-CE in suspect samples and (ii) accurate quantification of total EtO (targeted as 2-CE) in seeds and spices. Column no. 3 was employed for GC separation. Sample preparation strategy no. 1 used aqueous acetonitrile for 2-CE extraction and dSPE-based cleanup with Z-Sep+/MgSO4. In this way, fast screening of low levels of 2-CE in contaminated samples was enabled. Although free EtO was not determined by this strategy, the available results confirmed that “positive” samples always contain detectable 2-CE, in quantities fairly high compared to free EtO; in other words, this “bound” EtO was, practically without exception, the dominating component of residue. To determine EtO residue in line with the MRL definition, conversion of EtO to 2-CE was performed by acid hydrolysis in the presence of chloride ions. In this case, ethyl acetate was used for target analyte extraction from an aqueous acidic solution. To remove co-extracted, less polar compounds, including various plant pigments, a mixture containing PSA/C18/MgSO4 was employed.
For the maximum correction of matrix effects, D4-2-CE was used as an internal standard (see Figure 3).
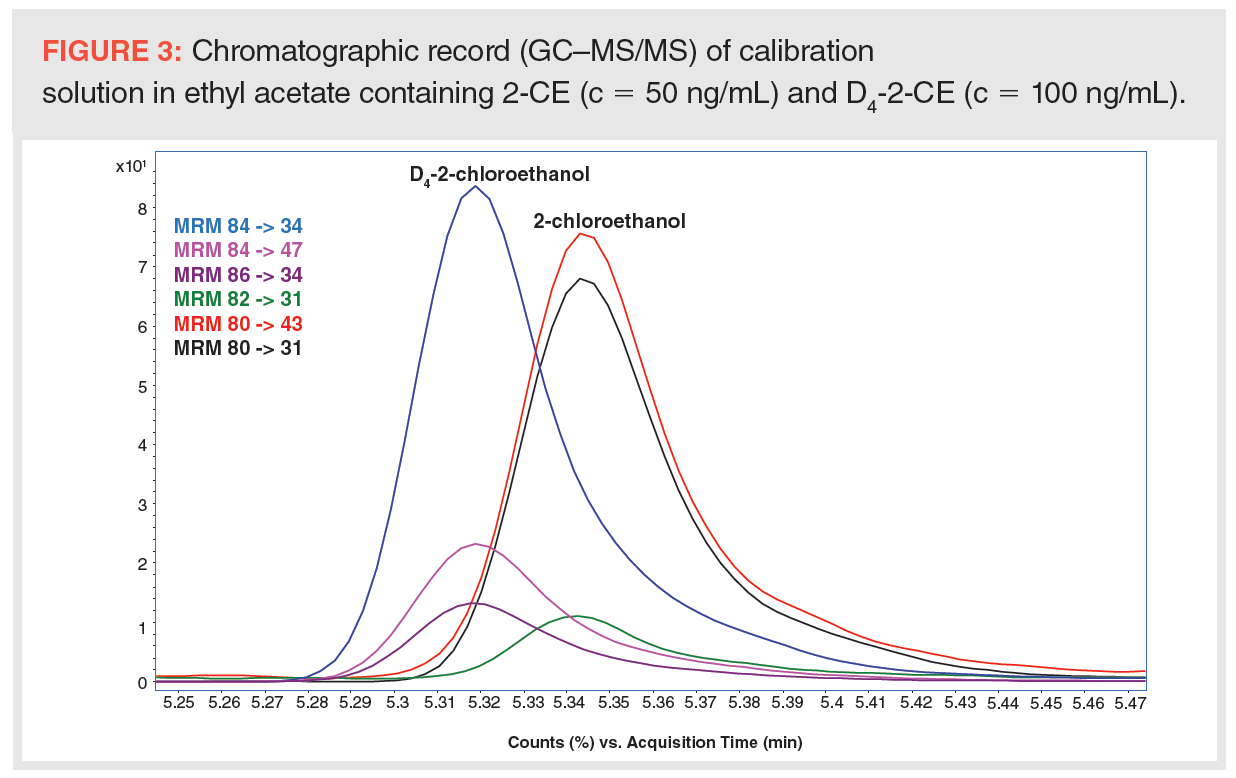
Table 1 summarizes the outcome of the validation of the latter strategy used for analysis of sesame seeds, ground pepper, and paprika spiked at levels 0.05 mg/kg for EtO and 0.012 mg/kg and 0.06 mg/kg for 2-CE. The recoveries ranged in tested matrices from 73 to 99%, from 101 to 119%, and from 86 to 109%, respectively. The repeatability (n = 6) expressed as relative standard deviation was in the range of 2 to 20%. This key performance characteristic met the criteria applied in the EU for the control of food and feed contaminants, according to the guidance document DG SANTE/12682/2019 (8).
To calculate recovery of the EtO conversion to 2-CE, six spiked (0.05 mg/kg) sesame seed samples were analyzed by acid hydrolysis followed by ethyl acetate extraction. Recovery of the EtO to 2-CE conversion was 50%, with repeatability at 11%.
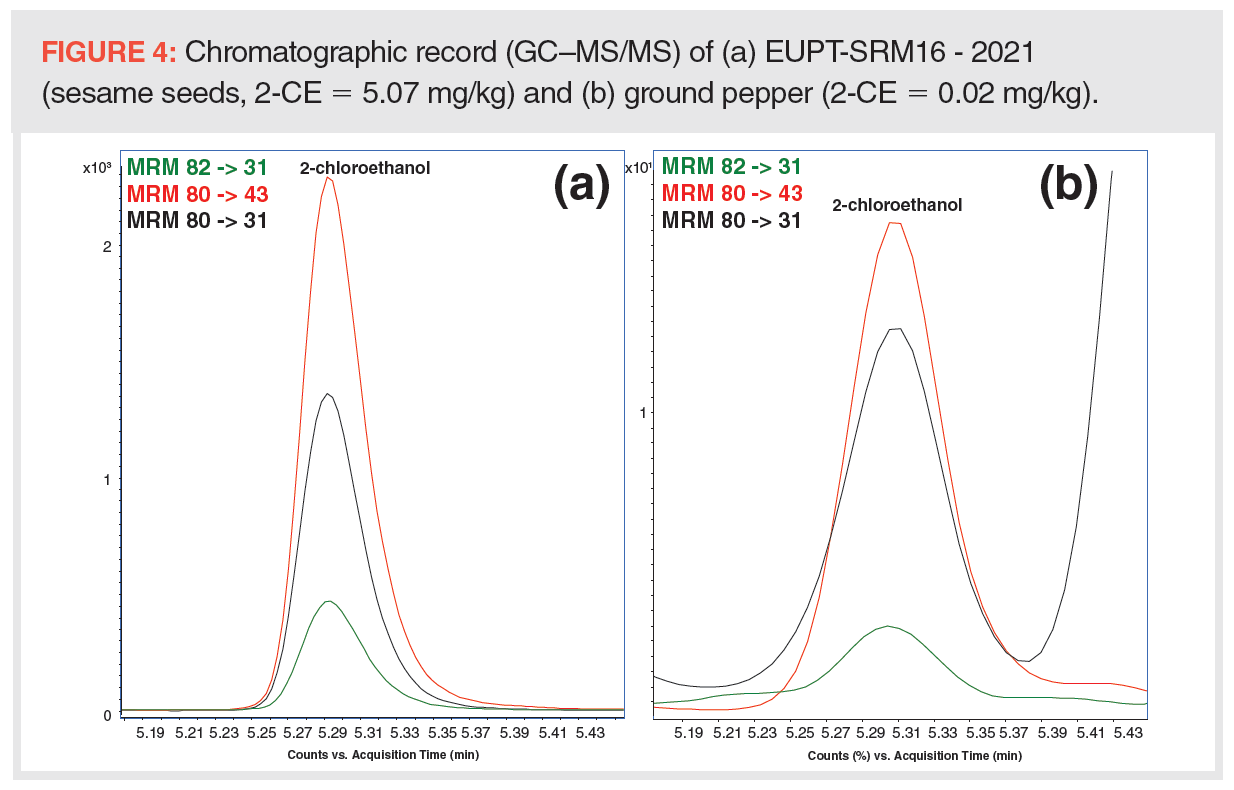
To demonstrate the accuracy of the generated data by this new GC–MS/MS method, EU proficiency test material, EUPT-SRM16 - 2021 (sesame seeds), was analyzed. Using classification based on the z-score for EtO (sum of EtO and 2-CE), a satisfactory result was obtained (z-score = 0.35). Figure 4 presents the chromatographic record of 2-CE in EUPT-SRM16 - 2021 at concentration level 5.07 mg/kg and 2-CE in ground pepper at concentration level 0.02 mg/kg.
Conclusion
This article has presented new implemented and validated analytical approaches based on GC–MS/MS for the determination of EtO and 2-CE in sesame seeds and spices. For rapid contamination control based on targeting 2-CE as a marker, the analysis of aqueous acetonitrile extract purified by dSPE can be used. To determine total EtO content, that is, both free and transformed EtO (in compliance with MRL definition, Regulation [EC] NO 396/2005), acid hydrolysis in the presence of chloride ions followed by ethyl acetate extraction and dSPE purification was the best choice. The accuracy of generated data by the latter method was proven through analysis of EUPT-SRM16 - 2021 test material (sesame seeds). All the performance characteristics were in line with guidance document DG SANTE/12682/2019 recommendations.
Acknowledgement
This work was supported from the grant of Specific university research – grant No A1_FPBT_2021_001
This work was supported by METROFOOD-CZ research infrastructure project (MEYS Grant No: LM2018100), including access to its facilities.
References
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC 2020 (consolidated text). Off. J. Eur. Union L 70, 1–16 (2005).
- J. Fowles, J. Mitchell, and H. McGrath, Food Chem. Toxicol. 39(11), 1055–1062 (2001).
- R.G. Liteplo, M.E. Meek, and W. Bruce, J. Environ. Sci. Health Part C 19(1), 219–265 (2001).
- S. Rebsdat and D. Mayer, in Ullmanns Encycl. Ind. Chem. Ed. (Wiley-VCH Verlag GmbH & Co. KGaA), a10_117 (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2001).
- W. GilsbachIand R.D. Weeren, Ringuntersuchungen Zur Validierung Einer Gaschromatographischen Methode Zur Bestimm. Von Rückständen Ethylenoxid 2-Chlorethanol Gewürzen Aus Paprika Chili 95(3), 83–90 (1999).
- K.G. Jensen, Z. Für Lebensm.-Unters. Forsch. 187(6), 535–540 (1988).
- M. Anastassiades and P. Schreiter, EUPT-SRM16, 2021, Test Item: Hulled Sesame seeds (Milled), https://www.eurl-pesticides.eu/library/docs/srm/SRM16_PreliminaryReport.pdf.,16 (2021), accessed 14 September 2021
- European Commission, Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed (SANTE/12682/2019; DG SANTE, Brussels, Belgium, 2019).
Michal Stupák is an assistant professor at the Department of Food Analysis and Nutrition, UCT Prague. His research is focused on the use of GC–MS in food quality, safety, and authenticity, as well as in natural products.
Maria Filatova is a PhD student at the Department of Food Analysis and Nutrition, UCT Prague. Her main focus is on GC–MS analysis of volatile compounds in food and natural products.
Vladimir Kocourek is a full professor at the University of Chemistry and Technology Prague (UCT Prague) and a quality manager of the accredited Metrological and Testing Laboratory, Department of Food Analysis And Nutrition.
Jana Hajšlová is a professor at UCT Prague. She is the head of ISO 17025/2018 accredited laboratory and also heads a research group concerned with separation science in the field of food/environmental analysis. She is the chair of a series of prestigious international symposia – Recent Advances in Food Analysis (RAFA, www.rafa2021.eu).

A Matrix-Matched Semiquantification Method for PFAS in AFFF-Contaminated Soil
Published: April 14th 2025 | Updated: April 14th 2025Catharina Capitain and Melanie Schüßler from the Faculty of Geosciences at the University of Tübingen, Tübingen, Germany describe a novel approach using matrix-matched semiquantification to investigate per- and polyfluoroalkyl substances (PFAS) in contaminated soil.
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.









