Flying High with Sensitivity and Selectivity:- GC–MS to GC–MS/MS
Mass spectrometry (MS) is the most powerful detector available for gas chromatography (GC). This article reviews the fundamentals of MS/MS and how they relate to MS as a detector for GC, then examines scenarios where use of GC–MS/MS can solve complex problems.
Mass spectrometry (MS), often termed mass selective detection, is the most powerful detector available for gas chromatography (GC). Multidimensional mass spectrometry (MS/MS) takes mass selective detection to another level on benchtop systems, offering both universal and selective detection along with low detection limits. In this installment of “GC Connections,” we review the fundamentals of MS/MS and how they relate to MS as a detector for GC. We see how using full-scan analyses can make the detector universal, and how, by using selected ion monitoring and multiple reaction monitoring, the detector can be so selective and noise-free that femtogram quantitative analysis is commonplace. We then examine some scenarios that should lead analysts to consider using GC–MS/MS to solve complex problems.
High sensitivity and selectivity are among the most important goals of any chromatographic method development or optimization process. Instruments, stationary phases, and detectors are usually chosen with one or both of these goals in mind. In gas chromatography, mass selective detectors (MSDs or mass spectrometers) have been used for decades to provide both high selectivity and high sensitivity. Capillary gas chromatography coupled to mass spectrometry (GC–MS) is a straightforward, yet powerful, coupling of the selectivity of GC with the high sensitivity and option of universal or selective detection of MS. Traditional GC–MS provides multiple dimensions of separations and low detection limits in benchtop or smaller instruments.
Before flying into the details of MS/ MS, we should briefly review some of the terminology specific to MS as a detector for GC. Mass selective detectors operate in two modes. The first mode is full-scan, in which spectra are continuously collected in quadrupole systems at rates usually up to 10–20 spectra per second, depending on the mass range selected. The second mode is selected ion monitoring (SIM), in which one or more individual ions are monitored. The data can be obtained in three forms:
- A total ion chromatogram (TIC) is the sum of all signals that reach the detector and is a demonstration of nearly universal detection. The full mass spectrum can be obtained at any point on the chromatogram.
- An extracted ion chromatogram (EIC) is obtained from the TIC by choosing one or more individual masses and extracting these from the full data set. This allows both universal and selective detection in a single experiment, since the ion chosen for analysis can be characteristic of a single compound or compound class.
- Selected ion monitoring (SIM) is obtaining a TIC in which the detector is set to monitor only one or a few ions. If a spectrum is selected from the TIC, it will only show the few ions that were selected when the experiment was set up.
There are several common GC–MS and GC–MS/MS instruments. Single dimension, classical GC–MS is mainly performed using quadrupole mass analyzers. Ion trap, a derivative of quadrupole instruments, and time-of-flight (TOF) are also used for specific analyses. Quadrupole-based systems are simpler and less expensive; GC–TOF-based systems offer the highest sensitivity and much greater mass precision and accuracy.
GC–MS/MS can be achieved through several configurations, with a wide range of capability and complexity. The most common of these is GC–triple quadrupole-MS (GC–TQMS), while GC–ion trap-MS (GC–ITMS) and GC-quadrupole time-of-flight MS (GC–QTOF-MS) are also available. A brief discussion of the evolution of ion trap and triple quadrupole mass analyzers over the years is provided in the brochure by Huebschmann (1). Professors Chris Enke and Rick Yost, inventors of the TQMS, have provided two excellent video interviews discussing the development of the technique in detail (2, 3).
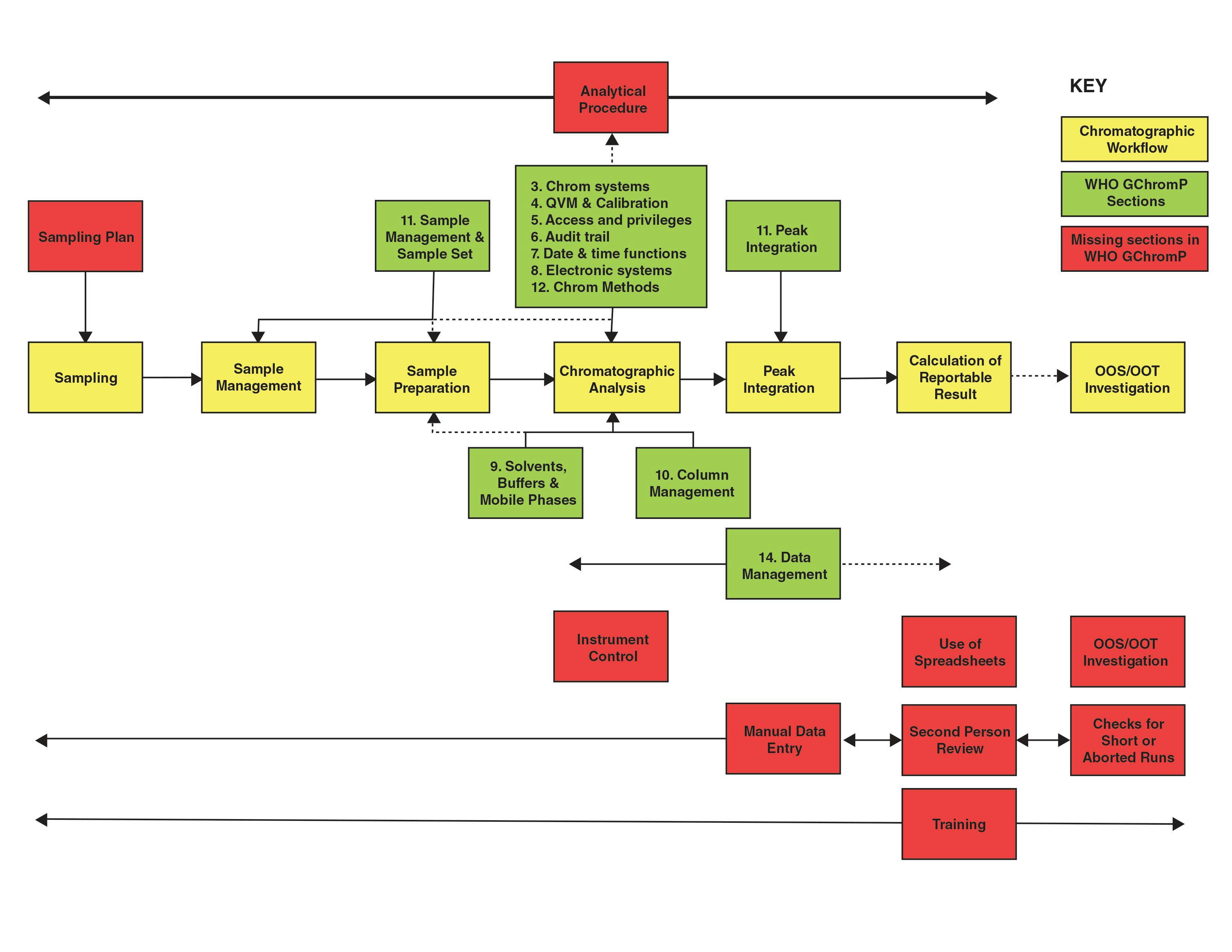
Figure 1 shows a block diagram of the detector on a GC–TQMS system compared to a traditional GC–MS system. Both are available in benchtop configurations. The main difference between the two systems is the presence of three quadrupole mass filters on the GC–TQMS system and one on the GC–MS system. Both systems use a transfer line with a capillary direct interface into the ion source and classical electron ionization ion source between the GC and the mass analyzer. Both operate with the ion source and mass analyzer at high vacuum, and use a classical electron multiplier to detect ions that pass through the mass analyzer. As described in more detail below, the first quadrupole (Q1) performs in the same manner as the single quadrupole in traditional GC–MS, selecting the ions that are ultimately passed to the electron multiplier detector. It can operate in either full-scan or selected ion monitoring modes. The second quadrupole (Q2) is used as a medium for collision induced fragmentation of ions passed through the first quadrupole to produce new fragments, and the third quadrupole (Q3) is used to select and analyze these new fragments.
FIGURE 1: Comparison of GC–MS and GC–MS/MS instrument configurations. (a) GC–MS and (b) GC–TQMS.
MS/MS is among the most flexible of all detectors, as it operates in several modes. In traditional GC–MS, full-scan MS provides a nearly universal detector; any analyte that can be ionized within the ion source can be detected. SIM-MS is a highly selective detector; the signals for the chosen ions are the only ones recorded. SIM is used for quantitation as the reduction in the signals being monitored versus full scan also reduces the noise, increasing the signal-to-noise (S/N) ratio, and therefore lowering the detection limit.
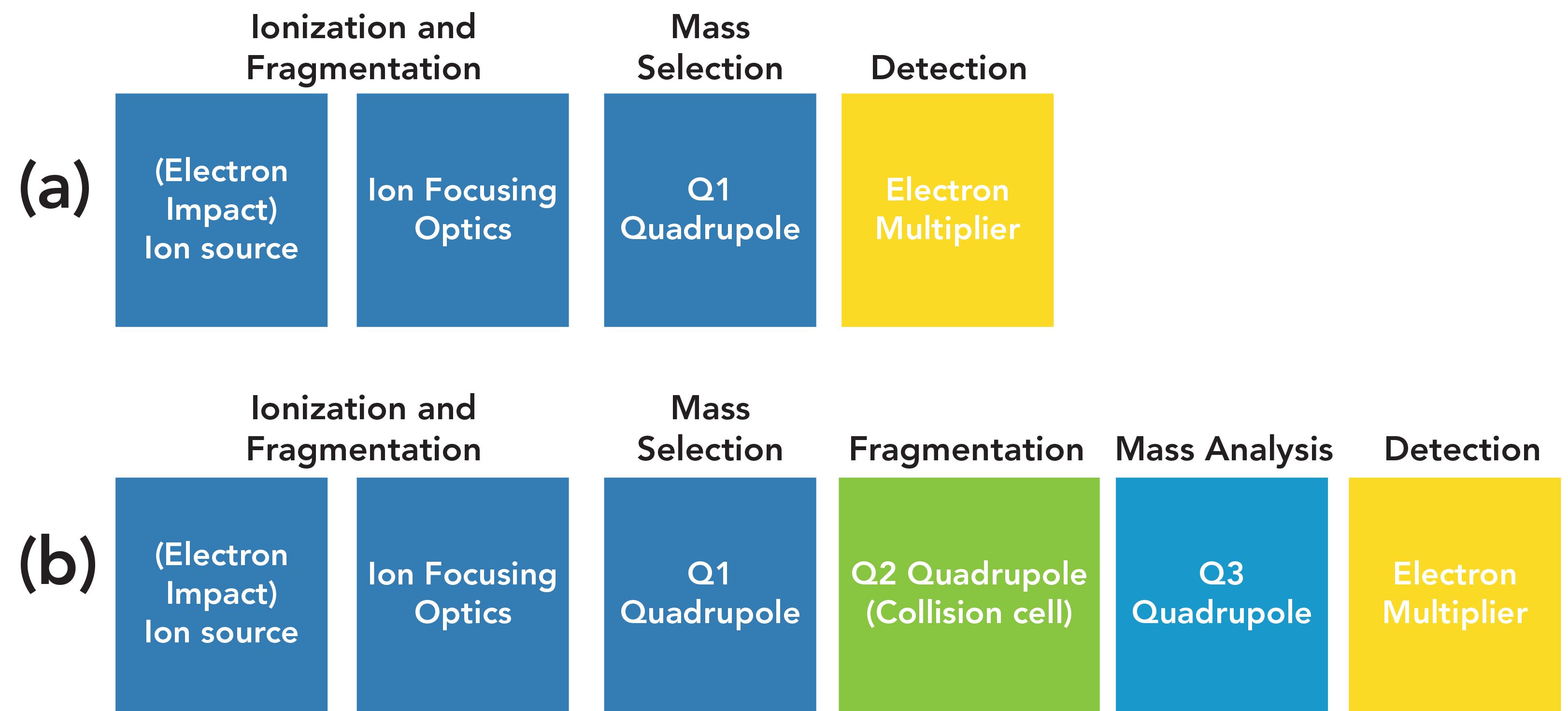
Figure 2 shows how the most common modes of quadrupole MS and MS/MS detection work. Full-scan and SIM single quadrupole detection are seen in the left side of the figure, in the ion source and Q1 images. The ion source generates ions including many masses; the quadrupole can either pass all of them (full scan) or selected ions (SIM). Triple quadrupole MS offers even more flexibility, since two additional quadrupoles are employed, as seen in Figure 2. Note that in both GC–MS and GC–MS/MS, classical electron ionization (EI) is by far the most commonly used ion source mechanism, so this is assumed in the following discussion.
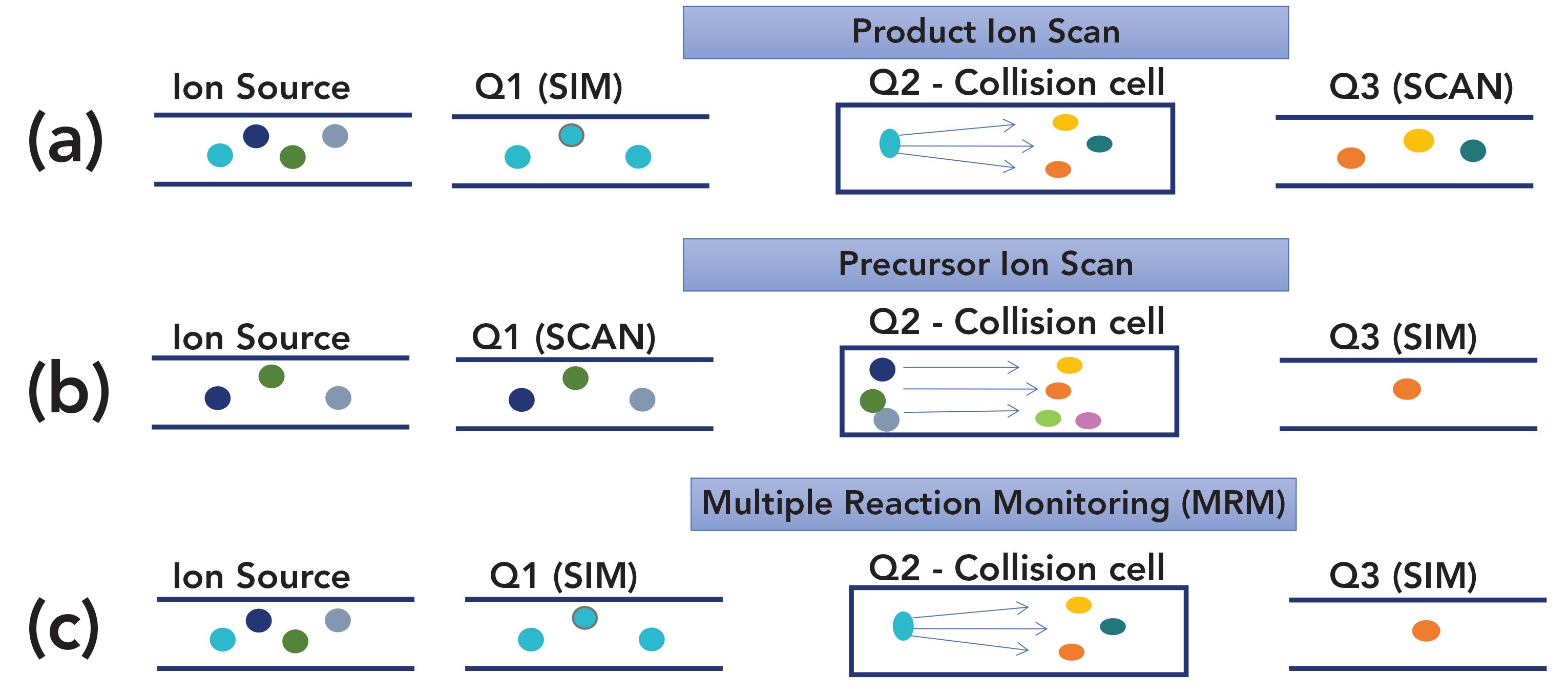
FIGURE 2: Modes of GC–MS/MS operation: (a) product ion scan; (b) precursor ion scan; and (c) multiple reaction monitoring.
A GC–MS/MS system can be operated exactly as a single quadrupole system. The second and third quadrupoles can be set to pass ions through without any further separation or reaction. This is often the first step in developing a method or transferring one to GC–MS/MS as it provides a traditional total ion chromatogram and traditional mass spectra of the analytes as a starting point.
In a product ion scan, the first quadrupole (Q1) can be set for selected ion monitoring, as in traditional GC–MS. Collision-induced ionization then occurs in the second quadrupole (Q2) to generate further fragmentation of the chosen ion, providing additional fragmentation that is analyzed by scanning the third quadrupole (Q3). This is a powerful tool for qualitative analysis since larger fragments from a traditional single dimension mass spectrum can be further fragmented to aid in confirming the correct structure. A product scan is also used for choosing transitions in initial method development for multiple reaction monitoring.
In a precursor ion scan, Q1 can be operated full scan, which passes all of the fragments generated in the ion source; think of traditional MS without the detector, with all of the resulting fragments reionized in Q2, and a single fragment chosen for monitoring in Q3. This is very similar to SIM in traditional GC–MS and is useful for quick quantitative analysis method development.
In multiple reaction monitoring (MRM), the most sensitive mode for quantitative TQMS, both Q1 and Q3, are set for single ion analysis. Based on the full-scan mass spectrum or a product ion scan, a fragment from Q1 is chosen and then, based on the further fragmentation in Q2 as seen by scanning Q3, a single fragment from Q3 is chosen. This provides possibly the ultimate selectivity as the possibility of two compounds, even closely related isomers, having the same transitions from precursor ion fragment to product ion fragment and the same (or close) retention time in the column decreases greatly. MRM also provides very low detection limits by significantly reducing noise in both dimensions.
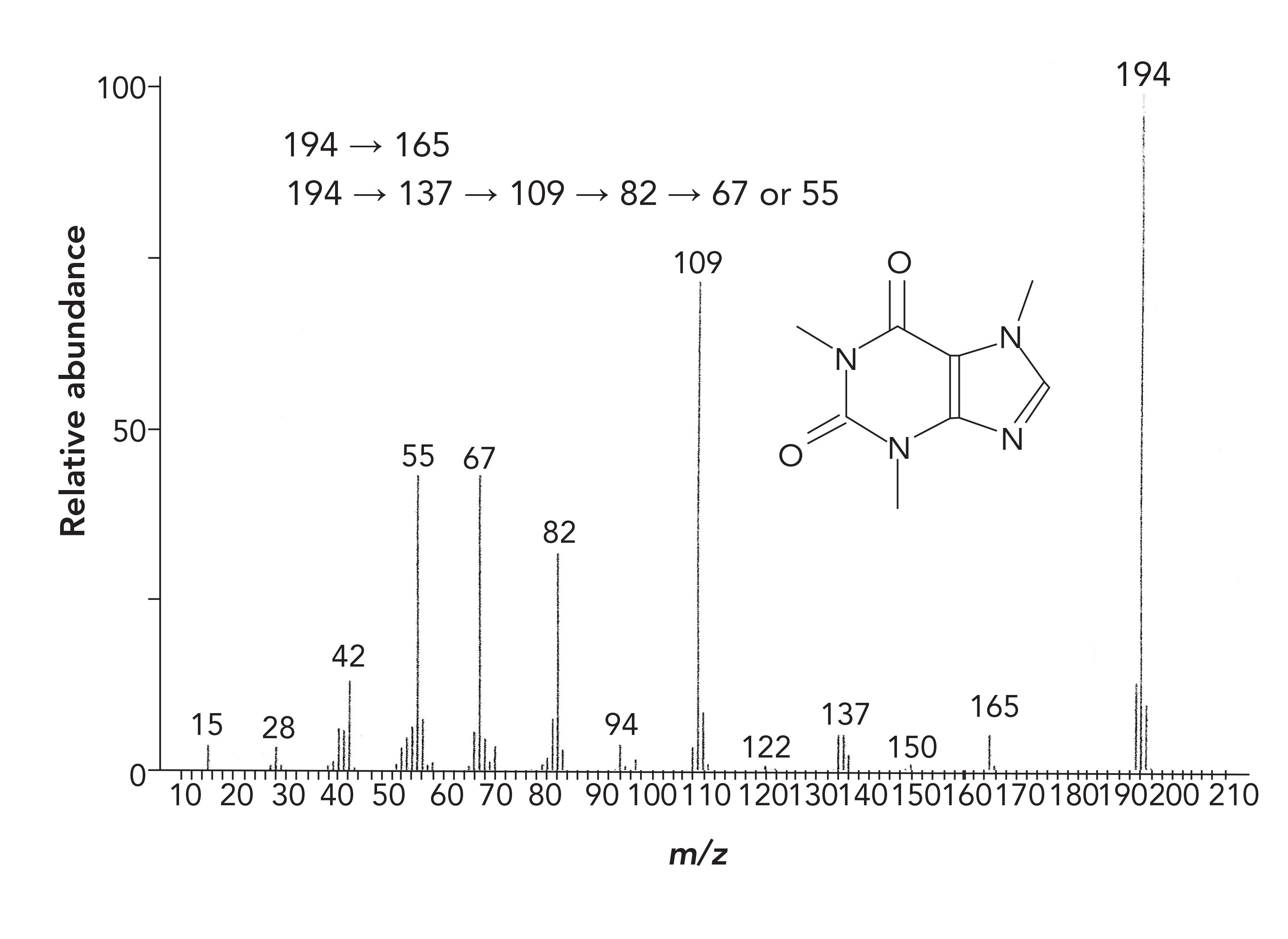
Figure 3 shows the well-known mass spectrum for caffeine, which provides an example for the utility and power of MRM. The mass transitions that generate the signals seen in the mass spectrum are also provided. In MRM, this full-scan spectrum is the starting point for method development, either determined experimentally or obtained from the literature. As this is an election ionization spectrum, it is good practice to interpret the spectrum, using traditional spectral interpretation rules at this point for a full understanding of the structural elements and decomposition reactions that generate each of the fragments. There are multiple excellent tutorial books relating to election ionization mass spectral interpretation available (4,5). Should there be additional spurious peaks, these can be identified at this point so they do not cause confusion later.
FIGURE 3: Full-scan mass spectrum and mass fragment transitions for caffeine.
In traditional single dimension GC–MS, the full-mass spectrum is used to identify the analyte. Quantitation is performed using either extracted ion chromatograms obtained by selecting one or more individual masses from the total ion chromatogram or by selected ion monitoring. SIM offers lower detection limits by reducing noise as most of the mass signals seen in the full-scan spectrum and their accompanying noise are eliminated.
In MRM, further advantage is taken of this noise reduction. One or more peaks from this initial mass spectrum is then chosen for further fragmentation. Usually this is the largest (base) peak, or it can be another strong signal that may be more characteristic of that compound. With this mass chosen, a second experiment is performed with Q1 operating in SIM mode to pass the one ion, termed a Precursor ion, with that ion being reionized in Q2, with the resulting new fragments, termed product ions, passed to Q3 operating in scan mode as seen in the Figure 2 product scan, to generate a mass spectrum of the fragment. One or more ions from this product spectrum can then be chosen for the final quantitative method. Any of the transitions seen in Figure 3, such as those from mass 194 to mass 109 are termed MRM transitions, and are often reported in the literature for completed methods. Any of the transitions can be used with one for quantitation and additional transitions for confirmation. Selectivity is generated because, instead of looking at individual masses that may not be unique to a compound, this is looking at transitions, which are very unique, especially when multiple transitions are used.
The ability to obtain spectra from precursor ions and to perform MRM leads to three situations in which GC–MS/MS is especially useful:
(1) Targeted analysis of a few analytes, in which you know the identity of the analyte or analytes, for which extreme sensitivity or low detection limits are required and/or the sample matrix is highly complex.
(2) Simultaneous targeted analysis of many analytes whose chromatographic peaks are not fully resolved.
(3) Untargeted analysis in which the matrix is complex, analyte chromatographic peaks are overlapped and the additional qualitative information about fragmentation is needed.
Analysis of extremely low levels of emerging contaminants in environmental water samples is an example of the first case. These appear at very low levels, and are the result of human activity. One application, freely available online shows analysis of several steroids in water at low parts per billion (ppb) and parts per trillion (ppt) levels using solid-phase micro-extraction coupled to GC–MS/MS (6). In this work, the sample preparation and detection were optimized but the chromatography was not, so it also illustrates the second problem: the analyte peaks are not resolved by the chromatography but are easily resolved using their differing MRM transitions. In a more extreme example, over 300 pesticides were extracted from apples and determined simultaneously in a single run using GC–MS/MS (7). This work illustrates the second and third cases: there are many analytes, the analysis can be either targeted or untargeted and the sample matrix is a complex food sample.
MS/MS provides the ultimate in detection for capillary gas chromatography. It can be both universal (full scan) or selective (SIM or MRM) and it is highly sensitive with detection limits of femtograms readily available. MS/MS is especially suited to the most difficult separation and detection problems. The trade off of this capability is capital and ongoing expense. MS/MS detectors are expensive, with fully loaded systems costing hundreds of thousands of U.S. dollars and have higher ongoing costs than traditional GC–MS systems, requiring special training to operate and needing additional maintenance compared to GC and GC–MS systems. The high sensitivity and low detection limit of MS/MS makes it especially sensitive to laboratory conditions such as clean carrier gases and careful sample preparation. Errors in sample preparation and contamination issues are often amplified when instrumental noise from the detector is lowered, so special care in sample preparation and laboratory management of GC–MS/MS is required. The unmatched combination of sensitivity with the ability to be both selective and universal makes MS/MS the most powerful detection tool available for capillary gas chromatography.
References
(1) H.J. Huebschmann, “Workhorses of the Chromatography Lab: Quadrupoles and Ion Traps,” Thermo-Fisher (Waltham, Massachusetts, 2014). http://tools.thermofisher.com/content/sfs/brochures/WP-10427-GC-LC-MS-Workhorses-Quadrupoles-Ion-Traps-WP10427-EN.pdf.
(2) A Look Back at the Birth of the Triple Quadrupole Mass Spectrometer (video), https://www.youtube.com/watch?v=whEO8kspM_g&vl=en Agilent Technologies (Santa Clara, California, 2017).
(3) One Man’s Noise: Christie G. Enke remembers development of the Triple Quad Mass Spectrometer (video) https://vimeo.com/102356325 Science History Institute (Philadelphia, Pennsylvania, 2014).
(4) F.W. McLafferty and F. Turecek, Interpretation of Mass Spectra, 4th Edition (University Science Books, Sausalito, California, 1993).
(5) R. Martin Smith, Understanding Mass Spectra, A Basic Approach (John Wiley and Sons, New York, New York, 2004).
(6) S. Chopra, P.F.C.L. Gomes, R. Dhandapani, and N. H. Snow, Scientia Chromatographica. 6(2), 105–116. (2014). https://www.iicweb.org/scientiachro- matographica.com/files/v6n2a02.pdf
(7) H.J. Schulte, H-U. Baier, S. Moreau, and K. Bollig, “Fast GC-MS/MS Analysis Of Multicomponent Pesticide Residues (360) In Food Matrix,” Shimadzu (Columbia, Maryland, 2015). https://www.shimadzu.com/ an/sites/shimadzu.com.an/files/pim/ pim_document_file/technical/white_ papers/12389/ego215070.pdf
About The Author

Nicholas H. Snow is the Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University, and an Adjunct Professor of Medical Science. During his 30 years as a chromatographer, he has published more than 70 refereed articles and book chapters and has given more than 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially gas chromatography, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and solid-phase microextraction. Direct correspondence to: amatheson@mjhlifesciences.com.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)