Flavour and Fragrance Analysis: Wondrous Vanilla
There are over 100 different types of vanilla, all characterized by different aroma profiles. To determine vanilla origin, and for quality control purposes, laboratories typically rely on headspace or thermal desorption techniques used in combination with gas chromatography–mass spectrometry (GC–MS). This article explains more.
There are over 100 different types of vanilla, all characterized by different aroma profiles. To determine vanilla origin, and for quality control purposes, laboratories typically rely on headspace or thermal desorption techniques used in combination with gas chromatography–mass spectrometry (GC–MS). Stephen J. Toth from the State University of New Jersey in the USA investigated this further in his doctoral dissertation to find a unified method to determine volatile and semi-volatile organic compounds in vanilla pods and extracts, and his work is discussed in this short article.
Photo Credit: Joe Biafore/Getty Images

The ancient Aztecs considered vanilla the "food of the gods" and today it is one of the most important and most widely loved spices in the world. There are more than 100 different types of vanilla, all of which belong to the orchid family. Originating from Mexico, vanilla is today mainly grown in Indonesia and Madagascar. Only Vanilla planifolia and Vanilla tahitensis are commercially relevant because they are the only orchid species to produce a commercially useful fruit.
One of the reasons why vanilla flavour is so widely used is that the key flavour compound can be synthesized from cheap ingredients such as paper pulp; however, real vanilla is very expensive with prices reaching as high as $80,000 per ton.1 The price of real vanilla is high because the amount of naturally grown and fermented vanilla does not even come close to satisfying world demand. Therefore, careful monitoring of quality and authenticity has to be performed to detect adulteration. This has traditionally been the responsibility of taste and flavour panels, but chemical analysis has increasingly come into play.
Most of us would not be able to recognize a vanilla plant or its fruit - the green, odourless, bitter-tasting vanilla fruit is nothing like the vanilla pods available in food stores. The fruit is transformed into dark brown, extremely tasty and flavourful vanilla pods over the course of a complex five-month-long fermentation process. The principal compound that characterizes vanilla flavour is vanillin, formed by the hydrolysis of glucovanillin. Controlling the fermentation process is key to producing the right flavour profile, taste, and overall quality product.
Fermented vanilla is marketed as either an extract in alcohol solution (extraction grade), or unprocessed (gourmet grade). A high-quality vanilla pod is characterized by a pleasant flavour and taste, with a moisture content of 18–25%, and a dark chocolate-like colour. The surface must be oily and free from defects and mold. Vanillin content should also be greater than 2%, but this is not always the decisive factor. Many types of vanilla can have vanillin content lower than 2% but still have excellent overall taste and flavour, leading to the conclusion that there are other flavour compounds that contribute significantly.1
As previously mentioned, producers of products that contain vanillin as a flavour ingredient can rely on standard chemical analysis for quality control; however, the analysis of real vanilla can pose a challenge. Because of the complexity of the chemistry and fermentation processes involved, it typically requires more than one analytical technique to provide reliable results. Stephen J. Toth from the State University of New Jersey in the USA, knows this from firsthand experience. Over the course of his work on his doctoral dissertation "Comparison and integration of analytical methods for the characterization of vanilla chemistry",1 Toth strove to find a unified analytical method for the determination of volatile and semi-volatile organic compounds (VOCs and SVOCs) in vanilla pods and extracts. The solution he found was to use two techniques: liquid chromatography (LC) and gas chromatography (GC) in combination with headspace and thermal desorption.
Determination of Vanilla Flavour Components
When determining semi-volatile or non-volatile flavour compounds in vanilla - such as vanillin, 4-hydroxy benzaldehyde, vanillic acid, and 4-hydroxy benzoic acid - high performance liquid chromatography (HPLC) is the separation method of choice. In his dissertation Toth referred to a large number of journal papers based on HPLC methods that served as reference points for his method development work, but in the end he used much shorter ultrahigh-pressure liquid chromatography (UHPLC) columns based on smaller particle size packing. This cut the analysis time for vanillin and related phenolic compounds from 13.45 min to 1.86 min (a sevenfold improvement in throughput), and reduced the amount of acetonitrile used by about two thirds. Still, for the wider range of more volatile compounds that are responsible for the bulk of vanilla's flavour, including many unknown compounds, HPLC is not the technique of choice. For these, gas chromatography combined with mass spectrometry (GC–MS) is used, preferably in combination with headspace (HS), headspace solid-phase microextraction (HS–SPME), or other combined analyte extraction-, concentration- and introduction-techniques.
To determine which technique was best suited for the analysis, Toth compared results from the following: SPME, headspace sorptive extraction (HSSE), and direct thermal desorption of deep-frozen ground vanilla pods. The flavour compound profiles of two bourbon vanilla pods were investigated. One was a perfect pod of good quality and the other had been rejected by a customer as unacceptable because of an "alcohol" off-flavour, thought to be caused by bacterial degradation products, including guaiacol formed by degradation of vanillin under anaerobic conditions.
Solid-Phase Microextraction (SPME)
Toth found that when reviewing the literature there were a large number of articles reporting on the analysis of vanilla extracts using SPME, particularly for polar compounds in alcohol extracts. Toth reasoned that this was because of the multitude of SPME phases available, the ease of automation, and the fast desorption of the concentrated analytes in the GC inlet. SPME has proven to be selective, efficient, and highly useful in practical analysis work when it comes to extraction of volatile compounds from the headspace phase.1 The main limitation of SPME lies in the limited phase volume (0.5 μL), reducing the sorptive capacity and sensitivity of the technique. Nevertheless, SPME was used to extract 35 compounds from vanilla, including contaminants from packaging as well as eight compounds that hadn't previously been identified in vanilla: 2-methylbutanoic acid; 4-hydroxybutanoic acid; heptanal; (E)-hept-2-enal; octanal; benzothiazole; dodecanal; and 5-methyl-2-phenylhex-2-enal.
Headspace Sorptive Extraction (HSSE)
Headspace sorptive extraction (HSSE) is a development of stir-bar sorbent extraction (SBSE). HSSE has been used for flavour analysis work involving a large number of different products2 and is based on using a stir bar coated with polydimethylsiloxane (PDMS) as a passive sampler in the headspace of a sample. In standard SBSE, the stir bar extracts analytes from a liquid sample while actively stirring it; in HSSE, the stir bar is positioned in the headspace above the sample from which it indirectly extracts volatile compounds. After equilibrium has been established between the liquid, headspace and stir bar sorbent phases, the stir bar is removed. Thermal desorption is then performed and analytes are transferred to the GC column. Because of the much larger phase volume of the sorbent (stir bar: 125 μL / SPME: 0.5 μL), the HSSE technique enables more efficient extraction of medium to non-polar compounds. Using HSSE, a total of four compounds were found and identified in vanilla for the first time: propyl acetate; heptanal; octanal; and (E)-3-hexen-1-ol acetate.
Dynamic Headspace (DHS)
Dynamic headspace is not a static technique relying on equilibrium between phases, but a dynamic technique that drives analytes out of the headspace and indirectly out of the sample using carrier gas. Purged analytes are then trapped and concentrated on an adsorbent trap at the purge outlet. In this case, Tenax-TA adsorbent was used in the trap. As opposed to SPME and HSSE, it showed no specific affinity for individual compound classes, but rather concentrated analytes over a wide polarity range. However, Toth found that compounds with three or less carbon atoms were generally not as efficiently trapped. Using DHS, he was able to extract and identify 24 compounds from the high quality vanilla, including nine compounds that had not yet been found in vanilla. The nine compounds were: 4-methyl heptane; 2,6-dimethyl-4-heptanone; 2,4-dimethyl-1-heptene; 4-methyl octane; 1,7-octadiene, 2,6-dimethyl; 3,7-dimethyl-1,6-octadiene; 4-octanone; 3-octanone; and (1,1-dimethylethyl)benzene.
Direct Thermal Desorption (DTD)
For direct thermal desorption, the sample was placed in a suitable inert glass tube (thermal desorption unit [TDU] liner) between two plugs of glass wool and placed in the sample tray. The TDU liner was transferred to the TDU by the autosampler. Thermal desorption/thermal extraction was performed using the following temperature programme: Initial temperature 30 °C – 60 °C/min – 275 °C final temperature.
Figure 1: Total ion chromatogram of a Tahitian Vanilla sample resulting from DTD-(TDU)-GC-MS analysis. The following compounds were found: 2-(5H)-furanone; 2-hydroxy-2-cyclopentene-1-one; 2-acetyl-2-hydroxy-gammabutyrolactone; 3,5-dihydroxy-2-methylpyrane-4-one; 3-phenyl-2-propenoic acid; 4-hydroxy-2-methoxy cinnamic aldehyde; 4-(4-hydroxy-3-methoxyphenyl)-3-butene-2-one (E); 2 isomers of 2-(4-hydroxy- 3-methoxyphenyl)-1,3-dioxane-5-ol; kauren; and z-12-pentacosene.
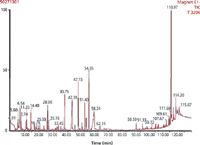
Desorbed analytes were cryogenically trapped and subsequently transferred to the GC column using a temperature programme. Direct thermal desorption using the TDU–GC–MS method enabled Toth to identify 74 compounds in both the "good" and "bad" vanilla samples. After further work, he found another 30 flavour compounds that had not yet been reported in vanilla pods. The most remarkable difference between the "good" and "bad" vanilla was in the vanillin concentration. The "good" sample contained 1.2%, whereas the "bad" contained only 0.1%. In the "good" vanilla samples, Toth further found high concentrations of acetic acid, 2-methoxy phenol, hydroxyl dihydromaltol, 5-(hydroxymethyl) furan-2-carbaldehyde, 4-hydroxybenzaldehyde, hexadecanoic acid, and 1-octadecanol. Toth reports: "The list of compounds identified in the good bourbon vanilla pod correlates well with previously reported data from the literature."
Compounds that he was the first to report in Bourbon vanilla pods included: acetone; 2-methyl propanal; 3-hydroxy-3-pentene-2-one; 2-(5H)-furanone; 2-hydroxy-2-cyclopentene-1-one; 4-hydroxy-5-methyl-3(2H)- furanone; furan-2-carboxylic acid (2-furoic acid); lilial acid, 4-(4-hydroxyphenyl)-3-buten-2-one; 4-(4-hydroxy-3-methoxyphenyl)- 3-butene-2-one (E); two isomers of vanillin glyceryl acetal; 1-octadecanol; ethyl heptadecanoate; ethyl octadecanoate; z-12-pentacosene; and z-14-nonacosene.
In the "bad" vanilla pods, Toth identified high concentrations of compounds such as: 2-methoxy phenol; 2-methoxy-4-methyl-phenol; hexadecanoic acid; and 1-octadecanol. Among the biggest differences uncovered by DTD of the "good" and "bad" bourbon vanilla pods were the loss of vanillin, increased concentrations of 2-methoxy-4-methyl phenol and 2-methoxy phenol, as well as the loss of hydroxyl dihydro maltol and hydroxymethyl furfural. Unlike the results from the analyses performed by SPME, HSSE, and DHS, the direct thermal desorption results did not include fusel alcohols, even though the rejected vanilla products did contain these compounds at different concentration levels. The presence of fusel alcohols is in itself a strong indication that bacterial degradation has taken place.
Each technique used showed both strengths and weaknesses, but when used in combination the techniques complemented each other well. Being able to draw on the whole range of headspace techniques - SPME, HSSE, DHS, and DTD - provides a critical advantage in terms of being able to cover all analytes while using solvent-free and highly sensitive extraction techniques.
References
1. S.J. Toth, Comparison and integration of analytical methods for the characterization of vanilla chemistry. Proquest, Uni Dissertation Publishing (2012).
2. Ray Marsili, Ed., Flavour, Fragrance, and Odor Analysis (CRC Press, Taylor & Francis Group, second edition, 2012).
Kaj Petersen, marketing manager at Gerstel since 2003, received his MSc from the Technical University of Denmark. Mr. Petersen has previously held positions as a research associate for Dow Chemical at Texas Tech University, as international application support specialist and as worldwide product manager for chromatography sample preparation instruments. Mr. Petersen is the author or co-author of a number of peer-reviewed publications.
E-mail: kaj_petersen@gerstel.de
Website: www.gerstel.com
This article is from The Column. The full issue can be found here: http://www.chromatographyonline.com/vol-10-no-20-column-november-06-2014-europe-and-asia-pdf


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










