Advances in Food Testing Using Core–Shell Technology
A brief overview of the advantages of core–shell technology for liquid chromatography (LC) separations with practical advice for chromatographers focused on developing or improving food testing methods is presented.
A brief overview of the advantages of core–shell technology for liquid chromatography (LC) separations with practical advice for chromatographers focused on developing or improving food testing methods is presented. The benefits of core–shell media across a wide range of LC platforms, the use of novel column selectivities such as biphenyl to obtain greater separation power, and how to implement core–shell columns into existing methods to achieve significant performance, productivity, and cost benefits will be demonstrated.
Global concern for adequate food testing is on the rise. As the requirements for testing change, laboratories around the world must improve their analysis to be as efficient as possible and to reach the required maximum residue limits (MRLs) dictated by their local regulatory agencies

Photo Credit: David Arky/Getty Images
Chromatographic improvements are one of the fastest growth areas. Traditional fully porous particles and phases have been sufficient for established regulated methods; however, with emerging hazards and the increasing size of analyte screening lists, challenges to improve productivity and selectivity of each analysis have arisen.
With particle technology advances, such as core–shell for high performance liquid chromatography (HPLC), ultrahigh-pressure LC (UHPLC), and LC coupled to tandem mass spectrometry (LC–MS–MS), laboratories are able to upgrade existing methods and move one step closer to discovering new contaminants and ensuring that our foods remain safe.
HPLC is a very popular analytical technique for testing food components. The key aspect of HPLC is the characteristics of the particles that are packed within LC columns, which in turn provide the separation needed to identify or quantify target analytes. Although traditional fully porous silica LC particles have been the standard choice for years, core–shell column use is increasing because it provides improvements for the analyst without the need to purchase new systems. In this article we will examine the general advantages of core–shell media for LC separations.
Core–Shell Particle — An Overview
What is core–shell particle technology? The current type of core–shell particles are monodispersed silica particles consisting of an impermeable silica core surrounded by a layer of fully porous silica. The primary distinction between core–shell particles and conventional HPLC media is the core–shell morphology. In contrast to traditional HPLC media, which consists of fully porous silica microspheres that typically exhibit a broad range of particle size distributions, core–shell media consists of a solid, impermeable silica core surrounded by an outer layer of fully porous silica. The benefits include improved resolution, decreased analysis times, and increased sensitivity.1
Columns packed with core–shell particles provide higher efficiency values than columns packed with fully-porous particles of the same diameter. This means that, all other things being equal, using columns packed with core–shell particles will result in narrower analyte peaks than columns packed with fully-porous particles of the same diameter. This means that the ability to resolve closely-eluting compounds is greatly increased. Note that backpressure is inversely proportional to the absolute particle diameter, independent of whether it is fully-porous or core–shell in morphology. Therefore, core–shell columns will generate the same pressure as fully-porous particles of the equivalent diameter, but with much higher efficiency levels.
Figure 1 illustrates the separation of a group of analytes under identical conditions. Figure 1(a) was obtained using a column packed with fully porous 3-μm particles while Figure 1(b) used a column packed with 2.6-μm core–shell particles for the exact same conditions. It is immediately apparent that the peaks are narrower for the results using the core–shell column. As the peaks become compressed, their height has also increased, as shown by the first peak, which has a 33% increase in peak height on the core–shell column. Because of the narrower peaks present on the core–shell column, the potential separation power is greatly increased. Note that the peak that was completely co-eluted in the black box becomes resolved as two distinct peaks on the core–shell column, shown in the red box.
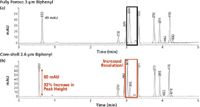
Figure 1: Coreâshell technology.
Of course, slight differences in selectivity may also contribute to the difference in this separation, but one can conclude that the two columns show remarkably similar selectivity overall, and that most of that improved resolution can be directly attributed to the increased efficiency of the column packed with the core–shell media. Analysts can then take advantage of this improved performance and use shorter core–shell columns to decrease analysis times and increase productivity.
Stationary Phases
LC stationary phase selectivities play an important role within food testing because of their ability to unequivocally identify target analytes for screening or confirmation. Figure 2 presents a spectrum of some of the stationary phases that are available within core–shell media — everything from highly useful C18 phases, to hydrophilic interaction liquid chromatography (HILIC) and high pH-stable phases. However, the biphenyl phase has become attractive to food testing laboratories because of its unique orthogonal selectivity compared to a traditional C18. So what is it that makes biphenyl so unique?
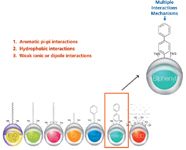
Figure 2: The selectivity of the biphenyl stationary phase.
The core–shell biphenyl is a stationary phase chemistry with a high degree of selectivity for polar and basic analytes (Figure 2), while also delivering the core–shell efficiency benefits mentioned previously.
The selectivity of the biphenyl is very distinct (orthogonal) from the typical C18 phases, which has been the standard for food testing. Analysts can use the core–shell biphenyl media when a standard C18 doesn't provide the right selectivity or performance. A good example of this involves large pesticides screenings in food samples. Typically these screenings include polar pesticides that are difficult or challenging to retain on typical C18s; however, by using core–shell biphenyl columns, polar pesticides can now be retained and results have been greatly improved.2
The selectivity of the biphenyl stationary phase (Figure 2) is based upon:
1. Aromatic pi-pi interactions — Between aromatic rings and pi electrons of target molecules and the double aromatic rings of the biphenyl ligand.
2. Hydrophobic interactions — Between the carbon skeleton of ligand and target analytes.
3. Weak ionic or dipole interactions — With the phenyl rings high electron density the biphenyl behaves almost as a weak cation exchanger, giving enhanced retention of many basic analytes.
Selectivity for Food Testing Applications — Method in Practice
Antibiotics in meats and other food sources are a growing concern and challenge for food testing laboratories. The wide range of chemical characteristics or classes ranging from polar, nonpolar, acidic, and basic properties makes it very challenging to screen larger sets in a single assay. Figure 3 provides an example of the blend of polar and hydrophobic retention properties of a core–shell biphenyl column for a broad range of antibiotics in an LC–MS–MS screening.

Figure 3: An example of the blend of polar and hydrophobic retention properties of a coreâshell biphenyl column for a range of antibiotics.
Method Parameters: Columns: 50 × 2.1 mm, 2.6-μm Kinetex C18 (Phenomenex) and 50 × 2.1 mm, 2.6-μm Biphenyl (Phenomenex); system: API 5000 MS + Agilent 1200SL; mobile phase: water with 0.1% formic acid, methanol with 0.1% formic acid; gradient: 0 to 100% B over 5 min, hold for 2 min; flow rate: 0.5 mL/min; temperature: ambient.
The analysis provided a rapid and effective separation of multi-class antibiotics in a single run.
One key to analysis with core–shell biphenyl media is the use of methanol in the mobile phase instead of acetonitrile. Technically the separation is not bad on the C18, but a couple of the peaks are not well retained and essentially elute in the void or very close to it. One would expect to have quantitation problems with these analytes because of matrix interference and ion suppression. With the exact same conditions on the biphenyl, the retention of those early-eluting components is dramatically improved, allowing the separation of these molecules from matrix interferences.
A further example is demonstrated in Figure 4. In this example neonicotinoid analysis is greatly optimized by switching the organic solvent in the mobile phase. When methanol is used, there is a dramatic difference in selectivity and retention with the biphenyl phase in comparison to the C18.

Figure 4: Analysis of neonicotinoids using (a) and (b) acetonitrile and (c) and (d) methanol in the mobile phase.
Optimizing Analysis in Food Applications
To achieve optimum analysis improvements, the following points should be considered:
1. It is important to choose the optimal core–shell particle size for the LC system. Selectivity is scalable across different particle sizes, so one can screen and do method development with any core–shell particle size, and then select the optimal particle size. Remember though, there is a balance between efficiency and pressure. In general, lower pressure equals longer column lifetimes and with older systems a larger particle size may be more appropriate. In general HPLC users can use the 5-μm or 2.6-μm particles, while UHPLC users can choose from the 2.6-, 1.7-, and 1.3-μm sizes.
2. Use methanol rather than acetonitrile to maximize the selectivity of core–shell biphenyl. Methanol allows the user to take advantage of hydrophobic, aromatic, and enhanced polar retention mechanisms available on the biphenyl core–shell particle. In many cases, when using methanol, the phase will deliver greater retention than a standard C18 phase.
3. Avoid strong solvent effects, particularly for early-eluting components. One very common mistake relates to sample solvents, and specifically problems with injecting strong sample solvents. Avoid injecting a sample solvent that is stronger than the mobile phase. All HPLC media is affected by this problem, but core–shell media is a bit more sensitive to these effects than fully-porous materials, primarily because the analytes tend be a little less retained, and therefore are more susceptible to solvent strength problems. In addition, early-eluting components are more strongly affected than late-eluters.
Conclusions
To conclude, core–shell particles and novel stationary phases, such as biphenyl, have become very desirable to food testing chemists globally. Productivity and selectivity gains are particularly important in an industry that depends more and more on complex screening lists. Although the particle technology and phase is the primary reason for the improvements, other items can also be considered to maximize impact on new and existing methods. Considerations such as particle size selection, mobile phase effects, strong solvent effects, and even sample preparation are items to be evaluated when working with these technologies.
References
1. Farcas et al., HPLC 2012 Conference.
2. André Schreiber, David Cox, and Lauryn Bailey, "Advanced Data Acquisition and Data Processing Workflows to Identify, Quantify and Confirm Pesticide Residues" NACRW 2014.
Jeff Layne earned his PhD in pharmacology from the University of Vermont (Vermont, USA). He has contributed to the development of a variety of Phenomenex HPLC media. He has developed and presented numerous technical seminars on aspects of HPLC media technology and HPLC method development worldwide. Dr. Layne is currently the Manager of both the Product Management and Technical teams at Phenomenex.
Simon Lomas graduated from the University of California at Los Angeles (California, USA) with a BS in microbiology, immunology, and molecular genetics and joined Phenomenex in 2004 where he has held several positions including Global Marketing Manager. He is currently Brand Manager for HPLC/UHPLC products.
Allen Misa holds a Masters of Science Degree in health care administration from California State University, Long Beach (California, USA) along with a microbiology degree from California State Polytechnic University, Pomona. He is the Industry Marketing Manager for Food and Environmental Markets for Phenomenex.
E-mail: JeffL@phenomenex.com
Website: www.phenomenex.com
This article is from The Column. The full issue can be found here:http://images2.advanstar.com/PixelMags/lctc/digitaledition/November07-2014-uk.html

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







