A Feasibility Study of Using Supercritical Fluid Chromatography (SFC)-UV-ELSD for Food and Beverage Analyses
The Application Notebook
Supercritical fluid chromatography (SFC) is a normal phase separation technique. In SFC, supercritical CO2 in combination with one or more polar organic solvents, most commonly alcohols, is used as the mobile phase. Owing to the lack of intermolecular interactions, supercritical fluid typically possesses lower viscosity and higher diffusivity than those solvents used in traditional high performance liquid chromatography (HPLC). This allows for higher flow rates, faster analyses, and the use of longer columns for higher chromatographic efficiencies. Initially deemed a niche chromatographic technique for chiral separation, the horizon of SFC applications has rapidly expanded to include achiral analyses of natural products, biodiesel, oligomer, pesticides/herbicides, and peptides. This is due, in part, to the improvements in detection choices and performances for SFC. Evaporative light scattering detectors (ELSDs) coupled with SFC have found wide use in many pharmaceutical and chemical laboratories (1).
Supercritical fluid chromatography (SFC) is a normal phase separation technique. In SFC, supercritical CO2 in combination with one or more polar organic solvents, most commonly alcohols, is used as the mobile phase. Owing to the lack of intermolecular interactions, supercritical fluid typically possesses lower viscosity and higher diffusivity than those solvents used in traditional high performance liquid chromatography (HPLC). This allows for higher flow rates, faster analyses, and the use of longer columns for higher chromatographic efficiencies. Initially deemed a niche chromatographic technique for chiral separation, the horizon of SFC applications has rapidly expanded to include achiral analyses of natural products, biodiesel, oligomer, pesticides/herbicides, and peptides. This is due, in part, to the improvements in detection choices and performances for SFC. Evaporative light scattering detectors (ELSDs) coupled with SFC have found wide use in many pharmaceutical and chemical laboratories (1).
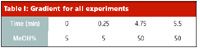
Table I: Gradient for all experiments
Food additives play a vital role in the modern food industry. They are used for various technical functions, including coloring, sweetening, and preservation. A rapid and reliable methodology to determine and quantify these additives is of great importance not only to the commercial value of the products, but also public safety. This was manifested by the media frenzy over the detection of Sudan I in chili powder and other spice products in Europe. To this end, SFC certainly holds great potential for the fast detection of adulterated products and can prevent their introduction into the food chain. Despite the popularity of SFC in pharmaceutical and related industries, reports on using SFC for food and beverage analyses are scarce. We report herein a preliminary feasibility study on using SFC-UV-ELSD in the area of food additive and beverage analyses. We prove in principle that SFC-UV-ELSD can be used for the fast separation and detection of sweetener and carbohydrates. A method to separate seven azo dyes by SFC in less than 2.5 min was also demonstrated, displaying a significant improvement in speed compared to HPLC.
Experimental:
Materials: Caffeine, glucose, fructose, ribose, sucrose, sucralose, and neohesperidine dihydrochalcone (NHDC) were purchased from Sigma-Aldrich (St. Louis, Missouri). Seven azo dyes, including Sudan I–IV, para red, methyl yellow, and lipid crimson were gifts from Dr. Dolak.
Instrumentation: Ten (10) μl of either chromatographic standards or food product solutions were injected into a Berger Analytix SFC-DAD-ELSD system (Thar Instruments, Inc., Pittsburgh, Pennsylvania). The hyphenated system consists of a Berger Analytix SFC, Agilent 1100 DAD, and a Polymer Labs PL-ELS-2100 and is controlled by Waters MassLynx software.
Chromatography: Two columns were used for the experiments: a Chromegabond Ethyl Pyridine column (4.6 × 250 mm, 4 μ, 120 Å) from ES Industries (West Berlin, New Jersey) and a Synergi Polar RP column (4.6 × 50 mm, 3 μ, 80 Å) from Phenomenex (Torrance, California). The system was held at 140 bar and 40°C with a flow rate of 5.2 mL/min for the Polar RP column and 3.0 mL/min for the Pyridine column. A gradient was used for all analyses (Table I). The Agilent 1100 DAD was scanning from 210–350 nm. For the Polymer Labs PL-ELS-2100, N2 gas flow rate was set at 1.45 SLM, nebulizer temperature was 50°C, and evaporator temperature was programmed to 30°C.
Results and Discussion:
High-fructose corn syrup (HFCS) is a mixture of fructose and glucose. Depending on the fructose/glucose ratio, the sweetness of HFSC can be compared to table sugar (sucrose), the disaccharide of fructose and glucose. HFCS is widely used in many processed foods and soft drinks in the US for economical reasons as well as longer shelf life. Neohesperidine dihydrochalcone (NHDC) is an artificial sweetener derived from citrus. NHDC is also known for its strong synergistic effect when used in conjunction with other artificial sweeteners.
Figure 1 shows the SFC chromatograms of a mixture of caffeine, fructose, glucose, sucrose and NHDC, a combination that can be found in many popular soft drinks. Analyses of a similar mixture using HPLC-UV-ELSD have been reported (2). As expected, only caffeine and NHDC were observed on the UV trace (Figure 1A). But all compounds were detected by ELSD (Figure 1B). The minor peak with a retention time of 1.55 min is likely an impurity in fructose. Figure 1 clearly demonstrates that this mixture can be separated by SFC and detected by ELSD in less than five min.
Figure 1
Sold under the trade name Splenda™ , sucralose is one of the most popular artificial sweeteners. Sucralose is approximately 600 times as sweet as sucrose (table sugar), but only 12% of the calorie content. Figure 2 shows the chromatograms of sucralose, sports drink A and B. Note that sports drink A was diluted ten-fold prior to the analyses and sports drink B was injected in its original form. The identification of the peaks is based on the retention time of the carbohydrate standards and the product label. It is evident that there is a dramatic difference in the sugar/sweetener content between the two drinks. Even after ten-fold dilution, there is abundant ribose, fructose, and glucose in drink A; whereas in drink B, the main component is sucrose with a noticeable amount of sucralose. This difference in sugar/sweetener composition results in the difference in calorie content. Drink B is marketed as a "low calorie" sports drink.

Figure 2
The final example is in the area of food adulterant detection, where speed of analysis is critical. Sudan dyes are oil soluble, azo dyes that are used in the textile and tanning industries. They have been classified as Class III genotoxic carcinogens and banned from use in food by European and Asian governing bodies since May 2003. Seven azo dyes were screened for detection and resolution on a Synergi Polar RP column. In our previous publication where a benzamide column was used (3), a narrow gradient (2–5% MeOH in 7 min) was employed to baseline resolve Sudan dyes I–IV. However, the narrow gradient could potentially limit the applicability of this methodology for food analysis, where sample matrices typically have diverse polarities. As shown in Figure 3, using a Synergi Polar RP column and a standard SFC gradient of 5–50% MeOH, all seven azo dyes were resolved within 3 min, representing a significant improvement in speed compared to HPLC and a more general applicability compared to our previous methodology.

Figure 3
Conclusion:
In this brief application note, we demonstrate the use of SFC-UV-ELSD for food and beverage analyses. ELSD can be easily coupled with SFC for the detection of compounds with no chromaphores, such as the carbohydrates presented in this study. The speed advantage of SFC, compared to HPLC, was clearly illustrated in all case studies and should be welcome by the food industries, especially for fast detection of food adulterants. Synergi Polar RP column displayed some unique selectivity compared to the popular 2-ethyl pyridine column for SFC. The general applicability of this column, however, calls for more comprehensive investigation.

Acknowledgments
The authors would like to thank Bill Farrell, Christine Aurigemma, and Loanne Chung of Pfizer (La Jolla, California) for permitting the use of their SFC-DAD-ELSD instrumentation.
References
(1) Lefler, J. LCGC Application Notebook. September 2006.
(2) Lucena R, Cárdenas S, Gallego M, Valcárcel M (2005) Anal Chim. Acta
530:283
(3) Dolak, L., Cole, J., Lefler, J. LCGC Application Notebook. February 2007.

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















