A Fast, Simplified Approach to Quantitative Headspace Analysis of Volatile Impurities in Drug and Packaging Products
Multiple headspace extraction (MHE) quantifies volatile impurities in condensed-phase samples for which matrix-matched calibration standards are difficult or impossible to prepare. However, it is costly for conventional chromatographic techniques because it requires multiple analyses of each sample. In contrast, application of direct-injection mass spectrometry—in the form of selected ion flow tube mass spectrometry (SIFT-MS)—can transform MHE into a cost-effective analytical approach because headspace analysis is faster and correlation of the first injection to full MHE analysis is stable for several weeks. This article demonstrates enhanced MHE workflows using styrene, formaldehyde, and N-nitrosodimethylamine (NDMA) analyses in polystyrene polymer, gelucire excipient, and ranitidine drug products, respectively.
Conventional, quantitative headspace analysis of volatile organic compounds (VOCs) emitted by condensed-phase samples largely relies upon the creation of matrix-match calibration standards to mimic complex partitioning. However, for certain matrices—such as polymers and gels—it is very difficult to prepare matrix-matched calibration standards. In these cases, quantitative determination of volatiles typically resorts to solvent extraction or stripping of all volatiles using dynamic headspace analysis (DHA), for example. As exhaustive extraction using DHA is a very long procedure, Kolb and Ettre (1) introduced the multiple headspace extraction (MHE) technique to achieve quantitative analysis via extrapolation of a limited number of headspace measurements that follow repeated headspace purge and regeneration cycles (Figure 1[a]). Example results are illustrated in Figure 1(b).
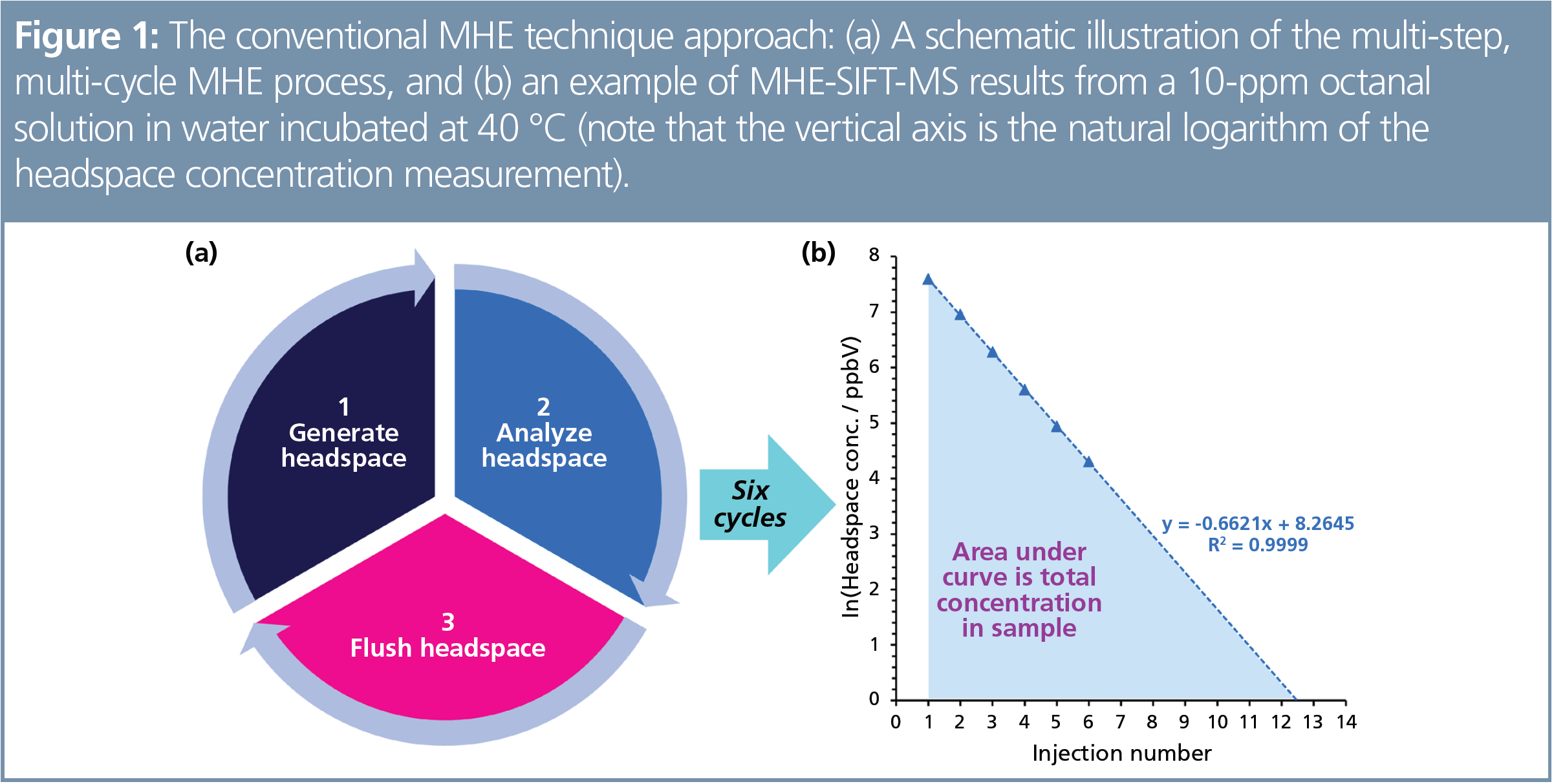
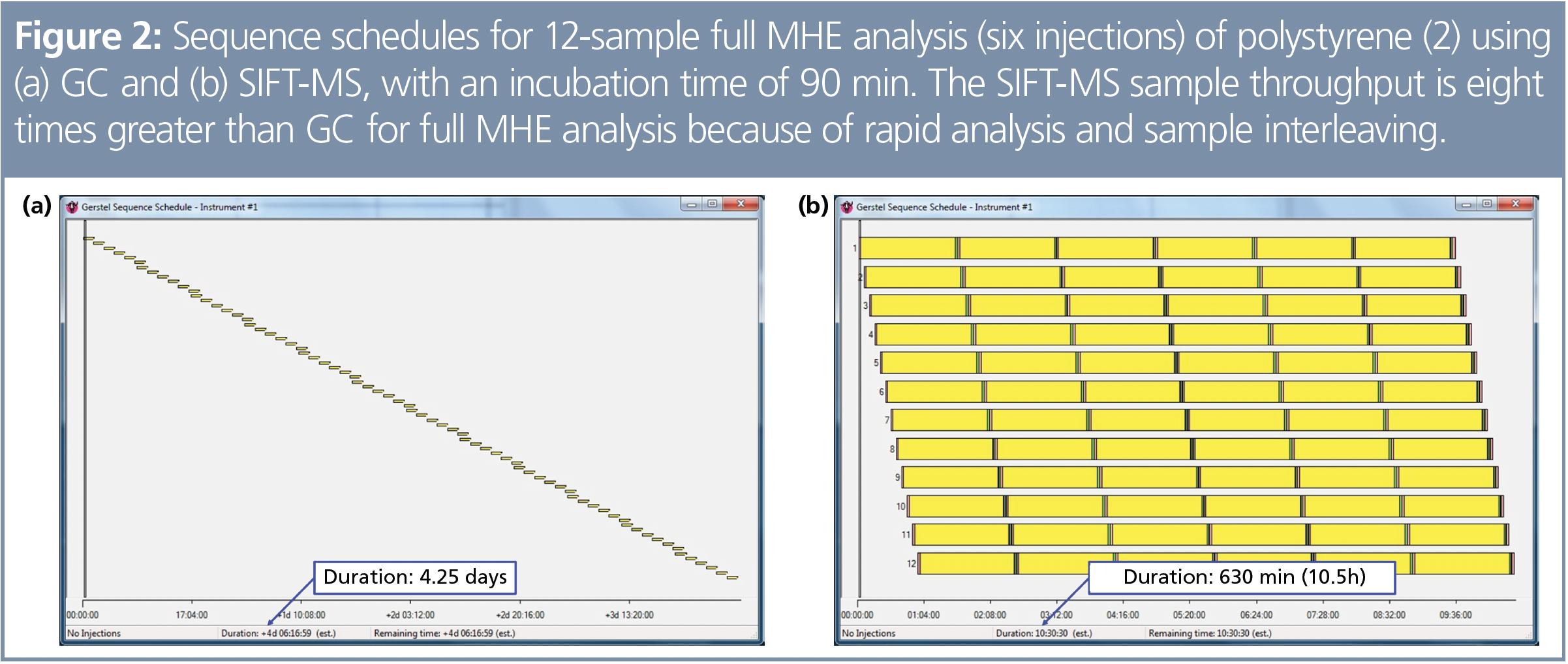
Relatively long sample run times in the original gas chromatography (GC) implementation of MHE mean that it is an expensive technique and is frequently avoided in routine analysis. Selected ion flow tube mass spectrometry (SIFT-MS) is, however, overturning this assumption. Direct static headspace analysis using SIFT‑MS can take less than two minutes, so significant efficiencies can be gained: one sample can be analyzed while the headspace is generated in up to 11 other samples (Figure 2) (2). The number of samples that can be analyzed in parallel is dependent on the time taken for the headspace analyze‑and-purge cycle relative to the optimized headspace generation time (the time to reach equilibrium). For polystyrene, an eightfold throughput enhancement has been achieved with SIFT-MS compared to the equivalent GC method (2).
This article reviews recent progress made in improving the MHE-SIFT-MS workflow to simplify quantitation of volatile impurities in drug products and packaging materials. Case studies presented include formaldehyde in gelucire excipient, N-nitrosodimethylamine (NDMA) in ranitidine products, and styrene in polystyrene polymer pellets. Reproducible, stable analysis using SIFT-MS can enable the MHE workflow to be transformed into a practical, quantitative approach.
Experimental
The SIFT-MS technique is described in detail elsewhere (3). Briefly, rapidly switchable reagent ions (H3O+, NO+, and O2+•) were utilized for gas-phase soft chemical ionization of trace volatile organic compounds (VOCs) in air and headspace. Real-time, high‑throughput analysis was achieved because SIFT-MS is chromatography‑free. Selectivity was achieved through the combination of multiple ionization mechanisms and mass spectrometric detection. A commercial SIFT-MS instrument was used in the studies reviewed here (either Voice200ultra or Syft Tracer models; Syft Technologies).
Automated headspace-SIFT-MS analysis is best achieved using autosamplers based on syringe injection (4). In this work, automated MHE analysis was performed using a SIFT‑MS instrument coupled with a multipurpose autosampler (MPS Robotic Pro, Gerstel) equipped with a purge tool (Gerstel). Details of analysis conditions are given in the cited literature. In general, headspace analysis was conducted using 20-mL headspace vials, with the sample extracted using a 2.5‑mL headspace syringe, followed by steady injection (usually at a rate of 50 μL/s) into a flow of nitrogen or zero-air make-up gas (10-fold dilution in the sample inlet). Optimized sample scheduling was achieved by the Maestro software package (Gerstel), as shown in Figure 2, which compares GC and SIFT‑MS.
Discussion
Synchronous (that is, real-time) sample analysis during headspace injection with automated SIFT-MS—in addition to faster sample analysis giving higher throughputs—facilitates faster MHE method development. With efficient sample scheduling (Figure 2), optimization of incubation conditions can be conducted rapidly (Figure 3[a]). Continuous headspace analysis (CHA)-SIFT-MS enabled the time for required headspace purge (Figure 1) to be optimized because the SIFT‑MS instrument can directly analyze the purge gas as it exits the headspace vial (Figure 3[b]). As described in reference 2, the throughput gain for MHE-SIFT-MS analysis was significant, making it a more cost‑effective approach per sample than MHE-GC.
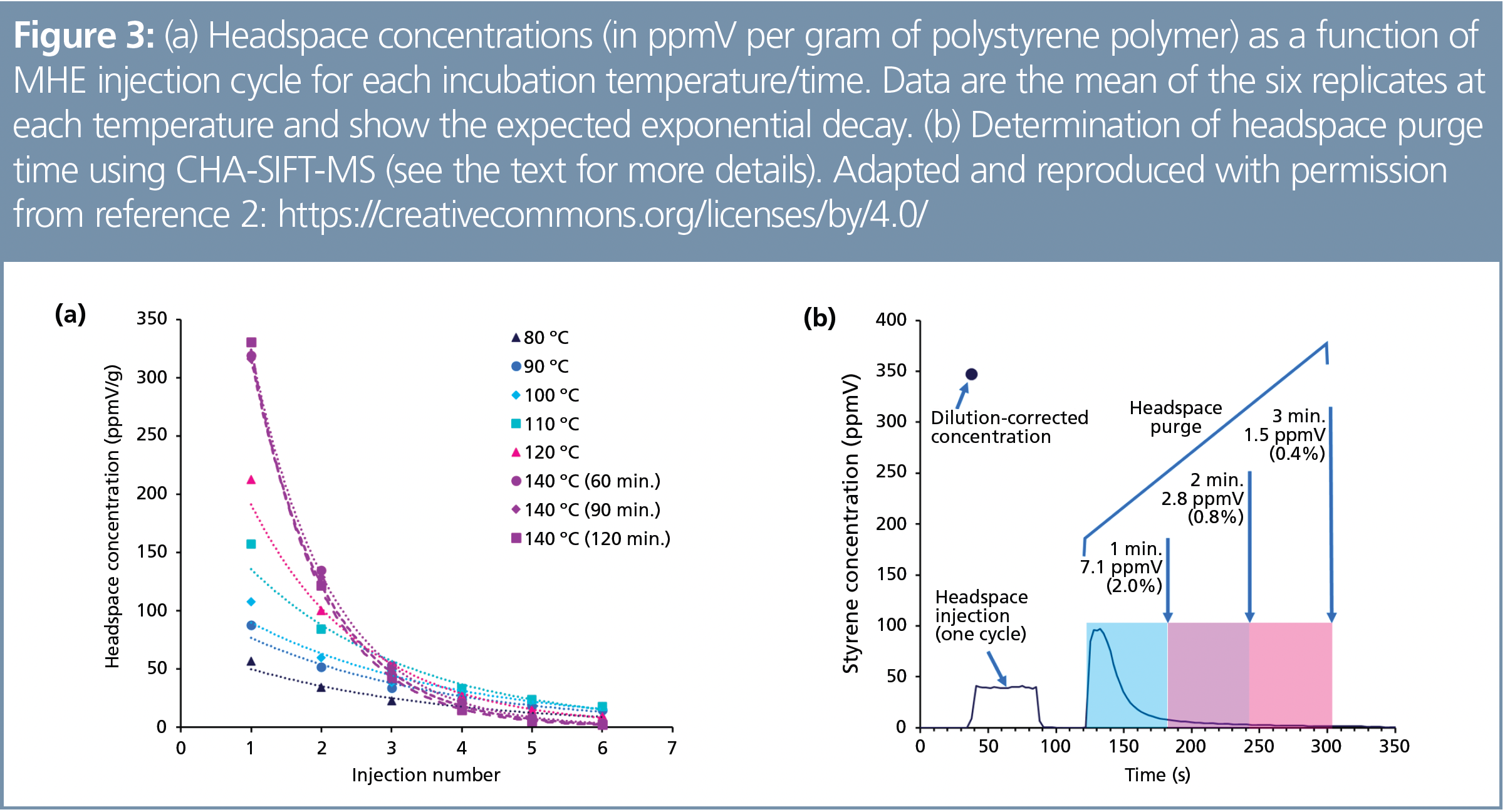
For routine static headspace analysis, automated SIFT-MS has demonstrated repeatable results (4–7). In reference 2, the repeatability of correlation of the first headspace injection with the full, six‑injection MHE analysis was demonstrated. At the optimal incubation temperature of 140 °C, repeatability better than 2.5% relative standard deviation (RSD) was observed (2).
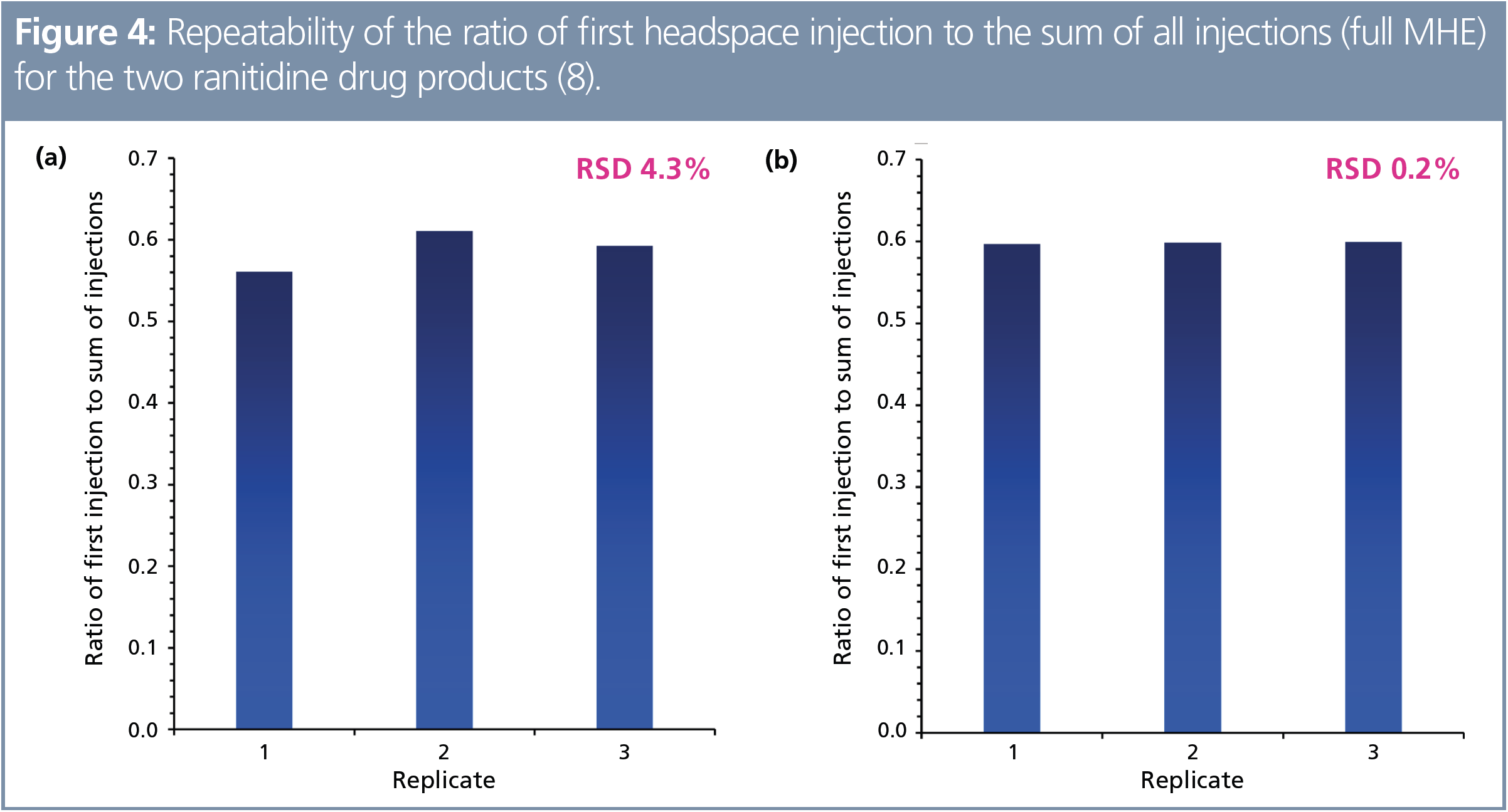
This approach was subsequently tested on a very different matrix: NDMA in powdered ranitidine tablets (8). Limits of quantitation (LOQs) in the very low nanogram range were obtained. Following full MHE method development, the NDMA concentrations in two products were 68 and 328 ng/g. Even in the presence of Class 3 residual solvents (isopropyl alcohol and ethanol), highly repeatable MHE calibrations were obtained (Figure 4), enabling headspace SIFT-MS analysis of powdered tablet to be analyzed directly without dissolution from a single headspace analysis at 12 samples per h (4). Furthermore, this analysis was achieved without any derivatization—the H3O+, NO+, and O2+• reagent ions all ionized intact NDMA in the gas phase. Hence minimal sample preparation and high sample throughput converge for the benefit of workflow.
Most recently, evaluation of the reproducibility and robustness of the MHE‑SIFT-MS calibration was undertaken to determine how frequently MHE “calibration” is required and how much samples can vary and calibration still apply (for example, in starting concentration).
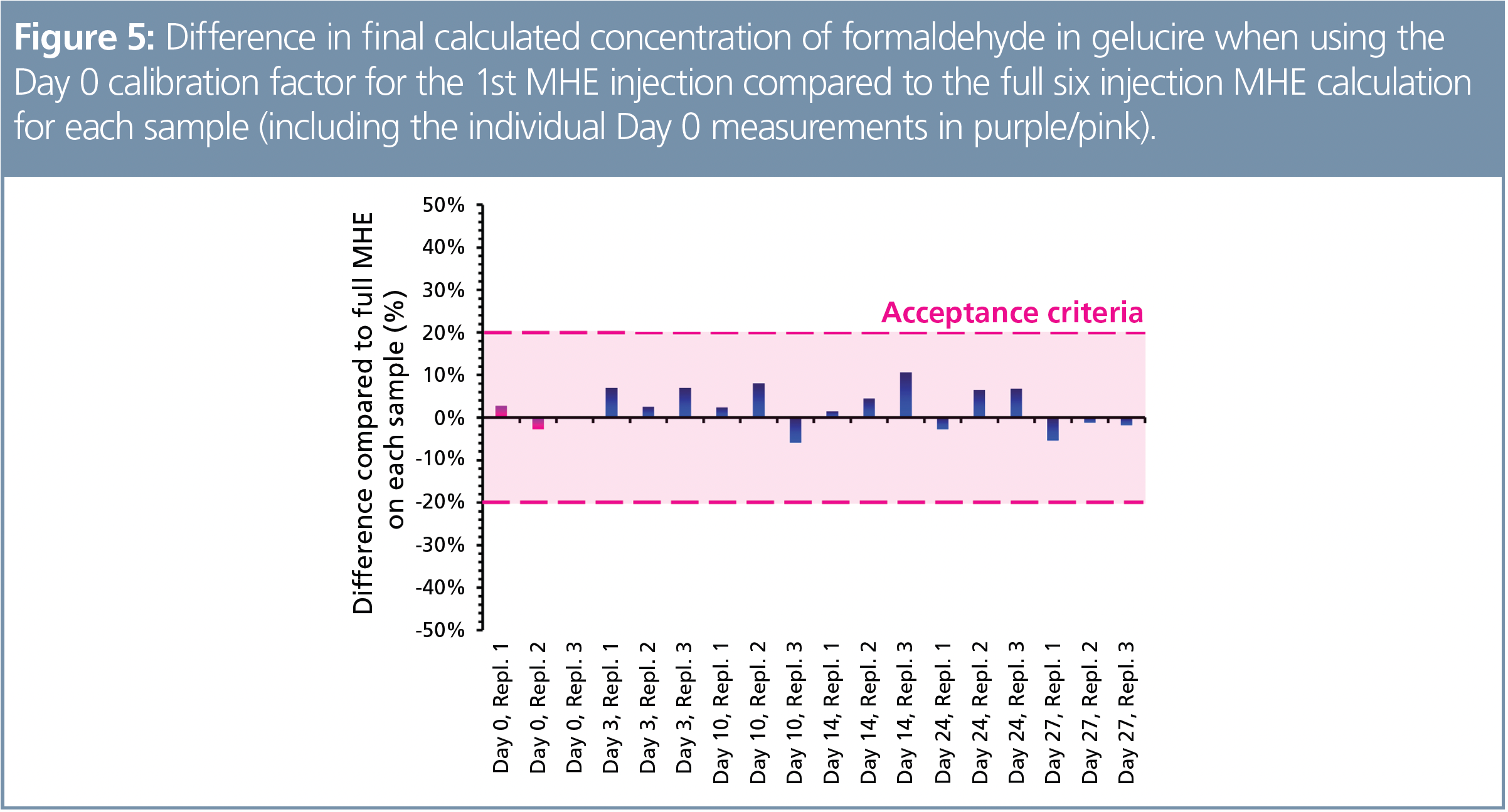
A challenging excipient matrix (polyethylene glycol-based gelucire 44/14) was utilized for the reproducibility study, while targeting formaldehyde—a chromatographically challenging and mutagenic impurity. As described elsewhere (9), when using SIFT‑MS formaldehyde was analyzed direct from the headspace with no derivatization or other sample preparation or preconcentration. Two independent studies (13 and 27 days each) were conducted to investigate the stability of the calibration factor—the instrument was not re-calibrated throughout the entire period. Full MHE was conducted in triplicate on each day that measurements were made. The results for the second study (with six measurement days) are summarized in Figure 5 (10). Here, the averaged “Day 0” calibration factor was used to calculate the formaldehyde concentration from the first injection of all replicates, and the difference compared with the individual sample’s full MHE result shown as a percentage. Acceptance criteria were based on the commonly used value of ±20% for volatiles analysis in the pharmaceutical industry. Clearly, the MHE calibration for formaldehyde in the gelucire 44/14 matrix held for at least four weeks, enabling quantitative analysis to proceed from a single headspace injection on any day within that period with no requirement for full MHE analysis/calibration, thereby maximizing sample throughput (12 per h).
The robustness of MHE calibration to concentration change was recently assessed (11) using C4 to C10 aldehydes in aqueous solution (with 2% sodium dodecyl sulfate [SDS] added to facilitate solubility) because this matrix enables all other variables to be fixed. Figure 1(b) shows one of the MHE plots from this study (11), in which the MHE calibration applies over a concentration of at least 1 to 2 orders of magnitude. This result enabled a given MHE calibration to be applied to a broader range of samples, further reducing calibration demand.
Conclusions
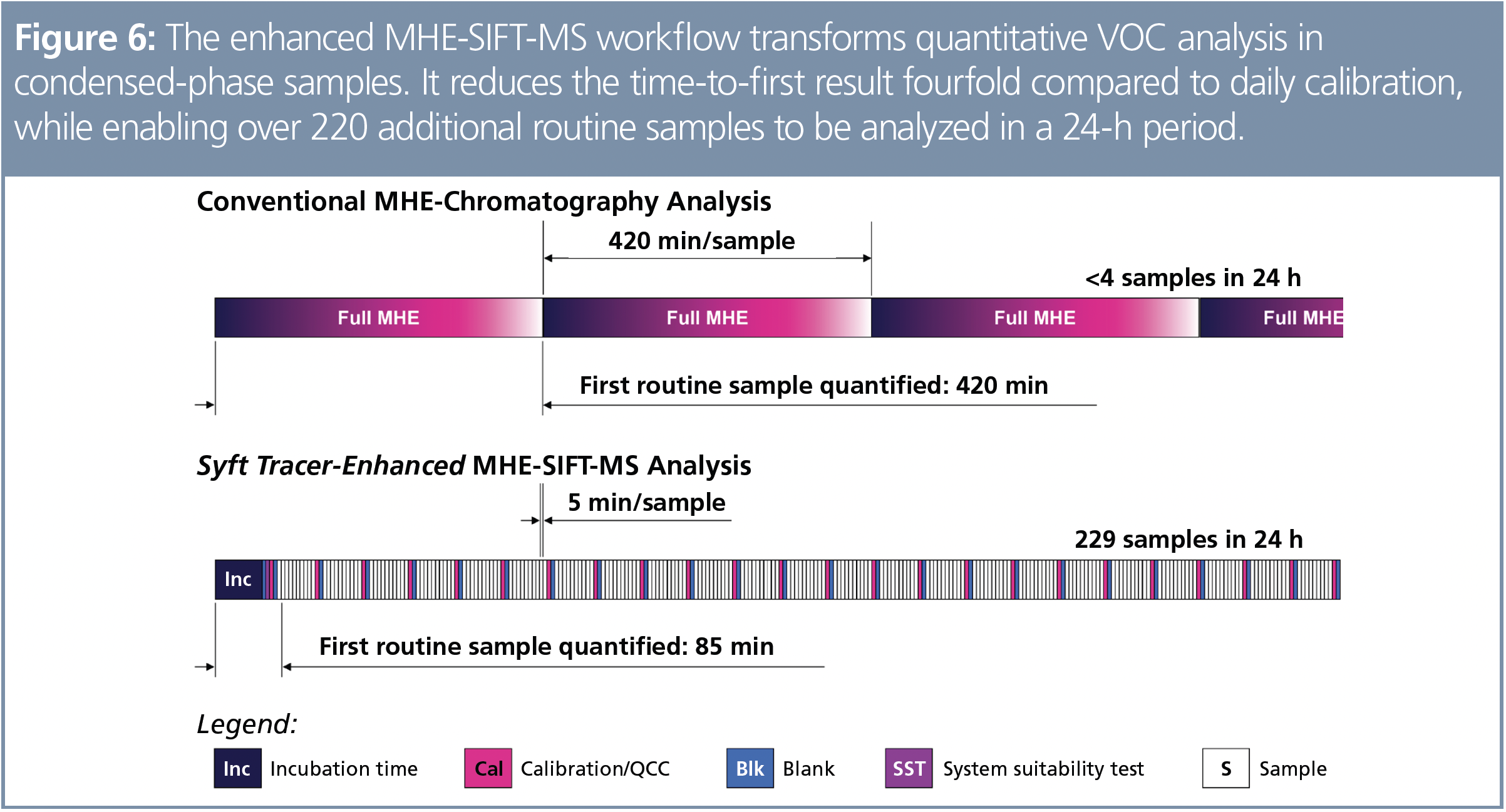
These examples demonstrate that MHE can be readily implemented with automated headspace-SIFT-MS. Through direct sample analysis, SIFT-MS can reduce sample preparation and shorten run times, enabling throughput to be accelerated. In addition, the unique stability of SIFT-MS instrumentation—in which diverse volatiles can be analyzed back-to-back without any configuration change (and hence need for recalibration)—underpins significant workflow improvements because calibrations can be conducted weekly or monthly. This is illustrated in Figure 6, where it is noted that routine system suitability tests and quality control checks are still included (and recommended).
In summary, quantitative headspace analysis of volatile impurities in condensed matrices has the potential to be transformed using automated-SIFT-MS analysis.
References
(1) Kolb, B.; Ettre, L. S. Static Headspace-Gas Chromatography – Theory and Practice, 2nd ed.; John Wiley & Sons, 2006.
(2) Perkins, M. J.; Langford, V. S. [MHE-SIFT-MS]. Part 1: A Protocol for Method Development and Transfer to Routine Analysis. Rev. Sep. Sci. 2022, 4 (1), e22001. DOI: 10.17145/rss.22.001
(3) Smith, D.; Španěl, P.; Demarais, N.; Langford, V. S.; McEwan, M. J. Recent Developments and Applications of [SIFT-MS]. Mass Spec. Rev. 2023, e21835. DOI: 10.1002/mas.21835
(4) Perkins, M. J.; Langford, V. S. Application of Routine Analysis Procedures to a Direct Mass Spectrometry Technique: [SIFT-MS]. Rev. Sep. Sci. 2021, 3 (2), e22001. DOI: 10.17145/rss.21.003
(5) Perkins, M. J.; Langford, V. S. Standard Validation Protocol for [SIFT-MS] Methods Applied to Direct Headspace Analysis of Aqueous [VOCs]. Anal. Chem. 2021, 93, 8386–8392. DOI: 10.1021/acs.analchem.1c01310
(6) Perkins, M. J.; Langford, V. S. Application of Headspace-SIFT-MS to Direct Analysis of Hazardous Volatiles in Drinking Water. Environments 2022, 9, 124. DOI: 10.3390/environments9100124
(7) Perkins, M. J.; Hastie, C.; Whitlock, S. E.; Langford, V. S. Pharmaceutical Residual Solvent Analysis: A Comparison of GC-FID and SIFT-MS Performance. App. Chem. 2023, 3, 290–302. DOI: 10.3390/appliedchem3020018
(8) Perkins, M. J.; Langford, V. S. Simple, Rapid Analysis of N-nitrosodimethylamine (NDMA) Impurity in Ranitidine Products Using SIFT-MS. Syft Technologies Application Note, 2022. https://bit.ly/3GB8wzW (accessed 2023-08-01).
(9) Langford, V. S.; Perkins, M. J. Demystifying Sample Preparation for Headspace Analysis Using Direct Injection Mass Spectrometry. The Column 2022, 18 (9), 24–28.
(10) Perkins, M. J.; Langford, V. S. Improved MHE-SIFT-MS Workflow: Even Faster Quantitation of Formaldehyde in Gelucire Excipient. Syft Technologies Application Note, 2023. http://bit.ly/431xTp2 (accessed 2023-08-01).
(11) Perkins, M. J.; Langford, V. S. Improved MHE-SIFT-MS Workflows: Concentration-Independent “MHE Calibration”. Syft Technologies Application Note, 2023. Available online: https://bit.ly/3CMYN82 (accessed 2023-08-01).
Vaughan Langford is a principal scientist at Syft Technologies in New Zealand. He joined Syft in late 2002 after completing his PhD in physical chemistry at the University of Canterbury, and postdoctoral fellowships at the Universities of Geneva, Western Australia, and Canterbury. He has 35 peer-reviewed publications, including seven book chapters or reviews, on a wide range of SIFT-MS applications, plus has contributed articles for industry publications and numerous conference papers.
Mark Perkins is a senior applications chemist and SIFT-MS expert at Element Materials Technology (formerly Anatune Limited), based in Cambridge, UK. Mark graduated from the University of Southampton, UK, with a PhD in electrochemistry. He was with the Malaysian Rubber Board’s UK research centre for 12 years, first as a senior analyst and then as head of the analytical section. He joined Anatune/Element in early 2015 in a role that supports and expands the analytical capability of SIFT-MS—with a particular focus on autosampler integration and the development of automated test methods.

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










