Fast Monoclonal Antibody Titer Determination with the TSKgel® Protein-5PW Column
The Application Notebook
The antibody therapeutics market is enjoying high growth rates, the major areas of therapeutic application being cancer and immune or inflammation-related disorders. Six of the top 10 best-selling global drug brands are monoclonal antibody-based. This market is predicated to show continued growth for many years to come, with more monoclonal antibodies (mAbs) designed and produced for treatments of specific diseases.
Early in mAb development there are many harvested cell supernatant samples which contain mAbs that are secreted by cell cultures. These samples must be screened for mAb titer; affinity protein A columns are often employed for this purpose. Determination of the optimal time for harvesting mAbs from cell culture supernatant and screening for the best cell clones that express the most amount of mAb are also accomplished using protein A columns. In addition, partial purification of mAb can be accomplished using an affinity protein A column initially to establish the right cell lines and to partially characterize a newly produced mAb. With many samples to be screened for different purposes, a reliable and high throughput column is needed for this workflow.
In this application note, the quick capture and accurate titer analysis over a wide concentration range of mAb is demonstrated using a TSKgel Protein A-5PW, 20 μm analytical column. Packed with hydroxylated methacrylic polymer beads coupled with a recombinant Protein A ligand (a code-modified hexamer of the C domain), this 4.6 mm ID × 3.5 cm PEEK column can be used with high flow rates for high throughput analysis and still maintains chromatographic efficiency, peak width, and resolution. In addition, the TSKgel Protein A-5PW column can perform for more than 2000 injections with no sign of deterioration and without cleaning.
Experimental HPLC Conditions
Results and Discussion
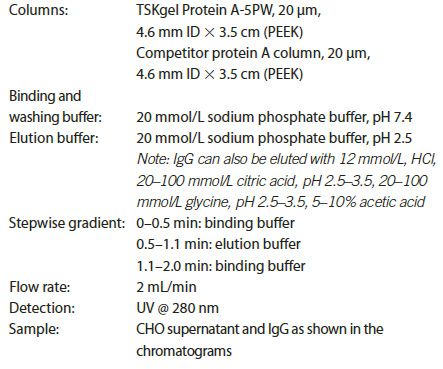
After harvesting, the supernatant from a CHO cell culture is briefly spun to remove cell debris and to concentrate the sample. It is then injected onto a protein A column for titer analysis. Figure 1 shows the fast capture of mAb (human IgG) using a TSKgel Protein A-5PW column. The run was completed within 2 min, including bind, wash, elution, and re-equilibration steps. Host cell proteins from the supernatant were not absorbed by the column and so eluted as a flow-through peak. Only IgG was captured and then eluted from the column at approximately a 1 min retention time. The IgG peak fraction was subjected to size exclusion chromatography using a TSKgel UP-SW3000 column for aggregate and monomer analysis. The result of that analysis indicated that the collected IgG consisted of more than 98% monomer (data not shown).
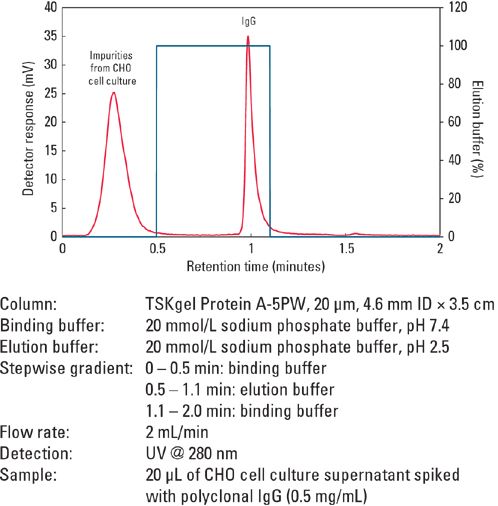
Figure 1: Fast capture of IgG in the mixture of CHO cell supernatant spiked with IgG using TSKgel Protein A-5PW column.
Determination of mAb concentration from harvested cell culture supernatant requires a column with good linearity over a wide dynamic range so that the concentrations of mAb can be accurately determined. Figure 2 is a calibration curve with good linearity (R2 >0.999) showing the wide dynamic loading range of the TSKgel Protein A-5PW column for a polyclonal IgG (0.1–10 g/L). Similar chromatograms from 2 to 200 μg without any change of peak profile or retention are produced by this column (Figure 3).
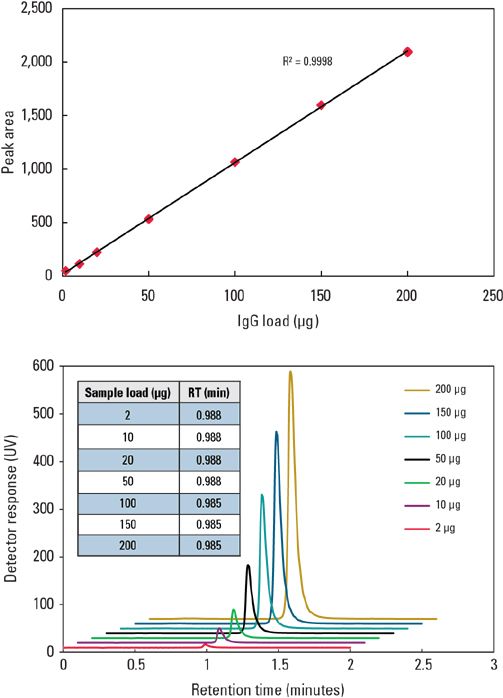
Figures 2 and 3: Wide range of loading concentrations of purified IgG for the TSKgel Protein A-5PW column.
Figure 4 demonstrates the high durability and again the wide dynamic load range of the TSKgel Protein A-5PW column. The column was subjected to a linearity analysis test. Purified IgG was initially injected onto the column with subsequent injections of IgG made at different volumes. The column was then used up to 2009 injections without being cleaned. A linearity analysis test was then repeated. No significant change in the calibration curve for IgG was seen. The column still maintained its high loading capacity with an excellent linearity (R2 = 0.9999).
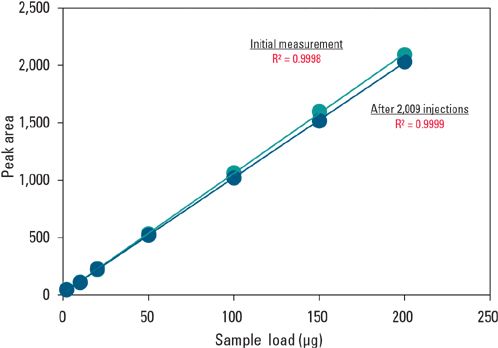
Figure 4: Durability and dynamic range of TSKgel Protein A-5PW column.
Four different flow rates (1, 2, 3, and 4 mL/min), were used to demonstrate the high flow rate performance of the TSKgel Protein A-5PW column. Figure 5 shows there is a minimal effect of flow rate on IgG binding or absorbing onto the column. The relative peak area percentages of the unbound (flow-through) protein peak and the bound IgG remained unchanged at different flow rates.
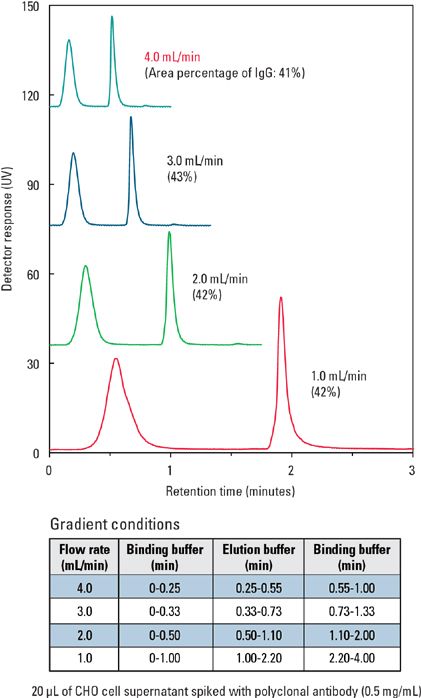
Figure 5: Varying flow rates used on TSKgel Protein A-5PW column to capture IgG.
Conclusion
The TSKgel Protein A-5PW column can capture and accurately quantitate monoclonal antibody from harvested cell culture media in less than 2 min. The wide range loading capacity of this column allows the titer of mAb at various stages of development to be determined. Because of the high flow rate tolerance and durability of the TSKgel Protein A-5PW column, high throughput analysis can be accomplished.
Tosoh Bioscience and TSKgel are registered trademarks of Tosoh Corporation.

Tosoh Bioscience LLC
3604 Horizon Drive, Suite 100, King of Prussia, PA 19406
tel. (484) 805-1219, fax (610) 272-3028
Website: www.tosohbioscience.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










