Exploring the Possibilities of High-Throughput Sample Preparation
LCGC North America
In sample preparation, what does "high-throughput" really mean?
Sample preparation has often been viewed as the bottleneck in analytical procedures. Surveys have shown that time is typically the most frequent problem area for sample preparation procedures and that analysts can easily spend a majority of the total analysis time on sample preparation. While newly developed extraction techniques address time, modern chromatography advances are also moving toward faster separations. Based on these considerations, what is high-throughput sample preparation? Do modern extraction methods adequately address the issue of time? How can we address the analytical need for speed?
The self-proclaimed world's greatest rock and roll band famously sang, "You can't always get what you want." It's easy to apply the Rolling Stones admonition to analytical sample preparation because surveys (1–3) have consistently shown that time, along with cost and solvent use, is among the most significant desires of analysts. Traditional sample preparation methods are often the rate-limiting step in the overall sample analysis process. We can envision three approaches for addressing the desire for high-throughput sample preparation: parallel sample processing, automation, and improvements in the process kinetics.
Evolution of High-Throughput Analysis from Microplates
Over the past several years, we've heard increasing calls for high-throughput sample preparation. But what is high-throughput sample preparation? It many cases, high-throughput stems from the high-throughput screening approach to combinatorial chemistry. This approach is the most established, based on the 96-well plate format. These microplates have been established for two to three decades. Key considerations for the acceptance of this approach are standardization of microplate dimensions and attributes by the Society for Biomolecular Screening and development of ancillary devices like repeating pipettes, vacuum manifolds, and more. Countless vendors are involved. Sample preparation and analysis using microplates includes liquid–liquid extraction (LLE), protein precipitation, solid-phase extraction (SPE), fluorescence, matrix-assisted laser desorption–ionization mass spectrometry (MALDI-MS), and separations.
Wells (4) presented a list of some of the uses of filtration microplates in pharmaceutical development, including
- clarification of acid, base, or organic digests of plant materials in medicinal chemistry,
- SPE of natural products,
- solution-phase synthesis in combinational chemistry using resin scavenger media,
- dye terminator removal,
- plasmid DNA binding,
- lysate clarification,
- membrane-based proteolytic digestion before MALDI-MS,
- filtration of precipitated proteins,
- filtration of plasma or serum samples in combination with direct injection techniques,
- filtration of reconstituted extracts, and
- solid-supported LLE, SPE, or exclusion chromatography.
As we can see, the development of 96-well plates and the major applications of this approach are in bioanalysis of liquid samples. This is because the transfer of liquid samples from analytical operation to another is somewhat straightforward. Recently, Scoffin (5) reviewed liquid transfer in high-throughput systems. These liquid-handling systems operate either by air displacement or positive displacement and can reliably work with microliter volumes of volatile or viscous liquids. Other combinations of liquid handling and sorbent-based extraction, including solid-phase microextraction (SPME), automated disposable pipette extraction, and microextraction by packed sorbent have also been used in high-throughput approaches (6,7). By combining these solvent-free, sorbent-based extractions with syringe-needle or pipette configurations, direct extraction and liquid transfer is accommodated.
Turbulent Flow Chromatography in Sample Preparation
In recent years, turbulent flow chromatography (TFC) has emerged as a high-speed extraction alternative for liquid samples. When flow rates increase significantly, the flow pattern shifts from a laminar, bullet-shaped profile to a turbulent profile. An example of this flat, turbulent flow profile is shown in Figure 1. Turbulent flow minimizes zone spreading and, most importantly for sample preparation purposes, facilitates radial diffusion (that is, mass transfer) to the stationary phase. Two years ago, sample preparation applications of TFC were discussed in this column (8). To achieve turbulent flow conditions, large, porous particles are combined with high flow rates. In general, 30–60 µm particles packed in 0.5–1.0 mm i.d. columns with flow rates greater than 1 mL/min and up to 6 mL/min achieve these conditions. Dramatic reductions in time are observed, while maintaining accuracy, precision, detection limits, and other figures of merit. Low-molecular-weight compounds readily diffuse into the porous particles, whereas larger compounds are less retained and are eluted to waste. Proteins are reported to be more selectively removed from biosamples using TFC than restricted-access media (RAM) or solid-phase extraction, as illustrated in Figure 2 (9). Thus, small molecule analytes in samples like whole blood, serum, plasma, or urine can be delivered to the injection port when coupled to high performance liquid chromatography (HPLC) in an on-line manner using column switching. Although minimal sample handling is necessary, resulting in fast analysis, the high flow rates necessary to achieve turbulent flow result in a large solvent consumption. Observed matrix effects require the use of standard addition. Recent reviews (10–13) have illustrated the use of TFC for biofluids, foods, and environmental samples.

Figure 1: Example of turbulent flow profile.
What About Solid Samples?
So far, we've focused the discussion of high-throughput sample preparation on liquid samples. The reasons are two-fold, as previously mentioned. Liquid handling is easier than manipulation of solids and the microplate technology drove the development of high-throughput methods. How can we make the analysis of solid samples high-throughput? Do the more recently developed extraction techniques take us in that direction?
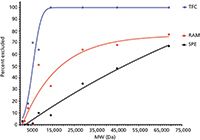
Figure 2: Comparison of turbulent flow chromatography (TFC), restricted-access media (RAM), and solid-phase extraction (SPE) cleanup columns as a function of the molecular weight excluded during sample cleanup. Adapted from reference 9.
During the time when 30–60 min chromatography runs were standard, Soxhlet extractions took 6–48 h. This bottleneck was even further exacerbated when spectroscopy or other rapid analytical procedures were used. During the past 20 years or so, several automated techniques (including supercritical fluid extraction [SFE], microwave-assisted extraction [MAE], and pressured liquid extraction [PLE]) have been developed, which provide the hope for high-throughput sample analysis. In addition to automation of the extraction procedure, these methods are characterized by very rapid extraction times compared with the traditional methods. However, simultaneously to the development of these techniques, advances in sensors, mass spectrometry, and chromatography have rendered even these modern technologies to remain the rate-limiting part of an analytical procedure. Sample preparation that takes 20 min, while several-fold faster than Soxhlet or shake-flask methods, is still slow compared with 5 min analyses. In the days of 6–48 h Soxhlet extraction and 30–60 min chromatographic analysis, the ratio of sample preparation time to chromatography run time was 6–96:1. However, it is common to perform, for example, 12 Soxhlet extractions simultaneously, so the ratio becomes 0.5–8:1. SFE, PLE, and MAE extractions commonly take 20 min, but have we really gained anything? It is now routine to perform chromatography with 10–30 min run times or faster. So the ratio remains 0.5–6:1.
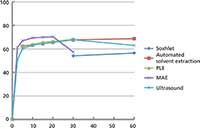
Figure 3: Relative percent of oil extracted from 725-µm coffee particles as a function of time (min) using various extraction processes. Adapted from reference 15.
The fundamental difference between extracting solid samples and liquid samples is the role of diffusion. Diffusion in liquids at room temperature is on the order of 10-5 cm2/s, while diffusion in solids at the same temperature is on the order of 10-9 cm2/s. But what about those solid extraction methods that operate at elevated temperatures? We have conducted a systematic comparison of SFE, MAE, PLE, and sonication extractions to examine the rate-limiting role of diffusion in these extraction methods. We extracted the oils from ground, roast coffee using these methods and determined the diffusion coefficients using the hot-ball model (14). Extractions were performed using dichloromethane at 100 °C under suboptimal conditions, which would allow the determination of the diffusivity. Soxhlet extraction is performed at some elevated temperature near, but below, the boiling point of 39.8 °C, while the modified Soxhlet approach includes leaching in boiling solvent as the main portion of the extraction. Ultrasonic extractions are performed in open-vessels, so the bulk solvent cannot exceed the boiling point, but on a molecular level temperatures during cavitation can reach 5000 K. PLE and MAE in this work were performed at 100 °C. Figure 3 displays the kinetic curves from these extractions (15). When the hot-ball model is applied to this data, the diffusion coefficients shown in Table I are obtained. The results show that analyte diffusion values, under similar extraction conditions applied across each method, were within the same order of magnitude. It seems unlikely that temperatures greater than those used in these techniques will be routinely applied for analytical extractions. Thus, when developing high-throughput extraction systems, the kinetics of extraction will not differentiate these alternative extraction procedures. PLE, MAE, and SFE seem to be the preferred approaches for developing high-throughput processing, but advantages with each technique will arise because of instrumental configurations.
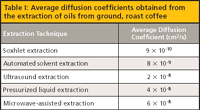
Table I: Average diffusion coefficients obtained from the extraction of oils from ground, roast coffee
Is High-Throughput Sample Preparation Necessary?
As discussed, the major approaches to achieve high-throughput sample preparation stem from parallel sample processing, automation, and the kinetics of the sample processing procedure. The success observed in the high-throughput sample preparation of liquids comes, in part, from automation and parallel processing. The microplates process 96 or more samples simultaneously. The instrumental approaches to extracting solids are also configured for automation and parallel processing. The kinetics of extraction is fundamentally limited by practical considerations. So perhaps the best approaches for high-throughput analysis will result from the integration of methods. Just as turbulent flow chromatography is coupled to liquid chromatography (LC), thermal desorption is coupled with gas chromatography (GC), and 96-well microplates are coupled to MALDI-MS, similar methodologies combining rapid sample and processing parallel approaches will begin to address this need. However, the analyst must also keep in mind what is truly desired. High-throughput sample preparation is not the ultimate goal, but rather high-throughput analysis. Chemometrics and other statistical approaches can glean the desired information from minimal analysis, and dilute-and-shoot or direct analysis mass spectrometry eliminate the need for sample preparation entirely, which is perhaps the fastest method of all.
References
(1) R.E. Majors, LCGC North Am. 9(1), 16–20 (1991).
(2) R.E. Majors, LCGC North Am. 20(12), 1098–1113 (2002).
(3) R.E. Majors, LCGC North Am. 31(3), 190–202 (2013).
(4) D.A. Wells, High Throughput Sample Preparation Methods and Automation Strategies (Elsevier, Amsterdam, The Netherlands, 2003), pp. 199–254.
(5) K. Scoffin, Am. Lab. 46, 17–19 (2014).
(6) J. Pereira, C.L. Silva, R. Perestrelo, J. Goncalves, V. Alves, and J.S. Camara, Anal. Bioanal. Chem. 406, 2101–2122 (2014).
(7) F. Mousavi and J. Pawliszyn, Anal. Chim. Acta. 803, 66–74 (2013).
(8) J.L. Herman, T. Edge, and R.E. Majors, LCGC North Am. 30(3), 200–214 (2012).
(9) O. Nunez, H. Gallart-Ayala, C.P.B. Martins, and P. Lucci, J. Chromatogr. A. 1228, 298–232 (2012).
(10) J. Pan, C. Zhang, Z. Zhang, and G. Li, Anal. Chim. Acta 815, 1–15 (2014).
(11) O. Nunez, H. Gallart-Ayala, C.P.B. Martins, P. Lucci, and R. Busquets, J. Chromatogr. B. 927, 3–21 (2013).
(12) M.D. Marazuela and S. Bogialli, Anal. Chim. Acta. 645, 5–17 (2009).
(13) L. Couchman, Biomed. Chromatogr. 26, 892–905 (2012).
(14) K.D. Bartle, A.A. Clifford, S.B. Hawthorne, J.J. Langenfeld, D.J. Miller, and R. Robinson, J. Supercrit. Fluid. 3, 143–149 (1990).
(15) J.L. Driver and D.E. Raynie, in preparation (2014).
Douglas Raynie is an Associate Research Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee.

Douglas Raynie
Ronald E. Majors "Sample Prep Perspectives" Editor Ronald E. Majors is an analytical consultant and is a member of LCGC's editorial advisory board. Direct correspondence about this column to lcgcedit@lcgcmag.com

Ronald E. Majors
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





