Evaporation-Free Extraction and Application in High-Throughput Bioanalysis by LC–MS-MS
The Application Notebook
Evaporation-free extraction (no drying down) is highly desirable because of its reduced cost and pollution, higher speed, and less possibility for contamination and conversion. Presented in this article are four types of evaporation-free extraction that are widely applicable. The first one is for the determination of didanosine over the range of 25.02–2502.00 ng/mL by injecting solid-phase extraction (SPE) eluate directly. The second is for the determination of betamethasone phosphate over the range of 2.51–250.60 ng/mL by injecting SPE eluate after pH adjustment. The third is for the determination of sumatriptan over the range of 0.99–99.40 ng/mL based upon SPE with high organic washing and low organic elution. The fourth is based upon automated dilution after protein precipitation for the determination of raloxifene-4'-glucuronide and raloxifene-6-glucuronide over the ranges of 2.02–202.40 ng/mL and 0.40–39.95 ng/mL, respectively.
Evaporation-free extraction (no drying down) is highly desirable because of its reduced cost and pollution, higher speed, and less possibility for contamination and conversion. Presented in this article are four types of evaporation-free extraction that are widely applicable. The first one is for the determination of didanosine over the range of 25.02–2502.00 ng/mL by injecting solid-phase extraction (SPE) eluate directly. The second is for the determination of betamethasone phosphate over the range of 2.51–250.60 ng/mL by injecting SPE eluate after pH adjustment. The third is for the determination of sumatriptan over the range of 0.99–99.40 ng/mL based upon SPE with high organic washing and low organic elution. The fourth is based upon automated dilution after protein precipitation for the determination of raloxifene-4'-glucuronide and raloxifene-6-glucuronide over the ranges of 2.02–202.40 ng/mL and 0.40–39.95 ng/mL, respectively.

Evaporation or drying down is used widely in the cleanup of biological samples by liquid-liquid extraction (LLE), solid-phase extraction (SPE), or protein-precipitation methods. Though it is necessary in some cases, such as to change solvent and to concentrate an analyte, there are several problems associated with the evaporation procedure. In addition to environmental pollution, evaporation and the subsequent reconstitution and transfer steps are time-consuming. These extra steps are subject to more potential contamination. Most importantly, evaporative–adsorptive losses or chemical reaction/conversion can occur during evaporation. For example, in a typical mixed-mode strong cation exchange–based SPE method, the elution solution is usually a mixture of a base and an organic solvent — for example, 10% ammonium hydroxide in methanol. The analytes and the other co-extracted metabolites in this strong basic media are then evaporated under heating at about 50 °C for 30–50 min. The high pH and heating could result in the hydrolysis or dissociation of glucuronides, acetate, phosphate, or decomposition of other labile metabolites (1). Therefore, developing evaporation-free extraction methods is highly desirable (2,3).
Though not named as such, several ways have existed to achieve evaporation-free LLE and SPE. In LLE, the traditional LLE with back extraction is one way. Matsui and colleagues (3) developed a mild clean-up procedure by using this approach to prevent the interconversion of R-donepezil and S-donepezil during extraction. Briefly, the two analytes were first extracted from a plasma sample to an organic phase. Then, 0.2 mL of dilute hydrochloric acid was added to the collected organic phase to transfer the analytes back to aqueous phase, which was then injected without evaporation. Though the procedure is straightforward, it would be quite tedious and labor-intensive when a sample batch is large. In addition, it is difficult to automate an LLE method with back extraction. One step further, directly injecting organic layer from LLE onto a reversed-phase column (4) or a hydrophilic interaction liquid chromatography (HILIC) column (5), were reported. Despite its use in special cases, the wide application of this approach (direct injection of organic phase from LLE) is limited due to the strong smell and corrosive and highly evaporative nature of common organic solvents, as well as its immiscibility with commonly used aqueous mobile phases in reversed-phase liquid chromatography (LC).
In SPE-based methods, Zheng and colleagues (6) reported an evaporation-free SPE method based upon automatic dilution of high organic SPE eluate by using custom-designed sample storage tubes with pierceable caps. Sanchez and colleagues (7) proposed direct injection of SPE elaute to an HILIC column without dilution and evaporation. Alternatively, evaporation-free SPE can be achieved through on-line SPE and column switching techniques, where samples are extracted on-line and the elution is loaded directly onto an analytical column. This is a highly automatic approach. However, special equipment is usually necessary. In addition, as the samples usually are processed sequentially, all the samples of one batch have to be present and kept at either room temperature or 4 °C for an unnecessarily extended period of time, which places stringent requirements on benchtop stability of analytes in matrix.
In recent years, we have developed and validated many different types of evaporation-free extraction methods. Most of these methods have been applied successfully to the high-throughput analysis of incurred samples at reduced cost and with improved reliability. Summarized in this article are four different types of evaporation-free extraction methods that generally are applicable without the need for specialized equipment. These extraction-free analytical methods can be used both manually and automatically. Their applications to the determination of didanosine, betamethasone phosphate, sumatriptan, raloxifene-4'-glucuronide, and raloxifene-6-glucuronide are described in this article.
Experimental
Chemicals and reagents: Raloxifene-4'-glucuronide, raloxifene-6-glucuronide, and their respective internal standards (raloxifene-d4-4'-glucuronide and raloxifene-d4-6-glucuronide) were all ordered from SynFine Research, Inc. (Richmond Hill, Ontario, Canada). Didanosine and its internal standard (2',3'-dideoxycytidine) were obtained from LKT (St. Paul, Minnesota) and Sigma (Oakville, Canada), respectively. Betamethasone phosphate and its internal standard (prednisolone phosphate) were purchased from USP (Rockville, Maryland). Sumatriptan succinate and its internal standard (bufotenine) were obtained from Euresian (Mumbai, India) and Cerilliant (Round Rock, Texas), respectively. Acetonitrile and methanol (Omnisolv), acetic acid (glacial, AnalaR), formic acid (AnalaR), ammonium acetate (AnalaR), ammonium formate (AnalaR), hydrochloric acid (Assured), and phosphoric acid (85%, AnalaR) were purchased from EMD (Toronto, Ontario, Canada). Ammonium hydroxide and Trizma base were obtained from Sigma. Human EDTA K2 or K3 plasmas were obtained from Valley Biomedical (Winchester, Virginia). Water was produced with Milli-Q water system (Millipore, Billerica, Massachusetts). High purity liquid nitrogen was supplied by Prodair (Mississauga, Ontario, Canada).
Calibration standards and quality control samples: All stock solutions were prepared in methanol except those of sumatriptan, which were prepared in water. The nominal concentrations of the stock solutions were 500, 500, 100, 200, and 40 µg/mL for didanosine, betamethasone phosphate, sumatriptan, raloxifene-4'-glucuronide, and raloxifene-6-glucuronide, respectively. All intermediate and working solutions were prepared by successive dilution of the stock solutions with methanol or water (for sumatriptan only). Calibration standards (CS1 to CS8) and quality control (QC) samples, including the lower limit of quantitation QC (LLQC) and the higher limit of quantitation QC (ULQC),) were prepared in control (blank) human EDTA K2 or EDTA K3 (for sumatriptan only) plasma.
Sample processing: The extraction of didanosine, betamethasone phosphate, and sumatriptan were all based upon SPE. Sep-Pak Vac C18 (200 mg/3 cc, Waters, Milford, Massachusetts), Oasis MCX mixed-mode strong cation exchange (30 mg/1 cc, Waters), and Bond Elut C8 cartridges (100 mg/1 cc, Varian, Mississauga, Ontario, Canada) were used in the sample processing of didanosine, betamethasone phosphate, and sumatriptan, respectively. The extraction of raloxifene glucuronides were based upon protein-precipitation. For details, refer to Figures 2, 5, 8, and 10 in the results and discussion section. A MultiPROBE II EX HT robotic liquid handling system (PerkinElmer, Shelton, Connecticut) was used in the sample processing of raloxifene glucuronides.
LC–MS-MS conditions: The LC system consisted of a solvent delivery module (Agilent series 1100, Palo Alto, California) and an autosampler (PE series 200 of Perkin Elmer). A Zorbax SB-C18 column (50 mm x 4.6 mm, 5 µm, Agilent) was used for both didanosine and sumatriptan while a Zorbax SB-C18 column (50 mm x 4.6 mm, 3.5 µm, Agilent) and a Symmetry Shield RP18 column (75 mm x 4.6 mm, 3.5 µm, Waters) were used for raloxifene glucuronides and betamethasone phosphate, respectively. The flow rate was 1 mL/min for all the analytes. Except for raloxifene glucuronides that were eluted in gradient mode, the other three analytes were eluted in isocratic mode.
For didanosine, the mobile phase was 15:85 (v/v) methanol–water with 3 mM ammonium acetate. The injection volume was 25 µL. For betamethasone phosphate, the mobile phase was a 30:70 (v/v) mixture of acetonitrile and 50 mM ammonium formate–0.05% (v/v) formic acid. An injection volume of 30 µL was used. In the determination of sumatriptan, the mobile phase was a mixture of 20:80 (v/v) methanol–water with 5 mM ammonium acetate. The injection volume was 20 µL.
Two mobile phases (A and B) were used in a gradient mode for raloxifene glucuronides. The mobile phases consisted of 20% (A) or 80% (B) of methanol (v/v), 1 mM ammonium formate and 0.1% (v/v) formic acid. In the first 0.5 min, 30% of mobile B was used, followed by a linear gradient to 100% of mobile phase B at 2.50 min, and then returned to 30% of mobile B until 3.50 min. The injection volume was 50 µL.
Mass spectrometry (MS) detection was carried out with a Sciex API 4000 MS system equipped with a TurboIonSpray interface (MDS Sciex, Toronto, Ontario, Canada). The ion source was operated in the positive mode for didanosine, sumatriptan, raloxifene-4'-glucuronide, and raloxifene-6-glucuronide and in the negative mode for betamethasone phosphate. The mass transitions used were m/z 237.2 → 137.1, 471.1 → 79.0, and 296.4 → 58.1 for didanosine, betamethasone phosphate, and sumatriptan, respectively. For both raloxifene glucuronides, m/z 650.3 → 474.1 was used. The Analyst software (version 1.4.1, MDS Sciex) was used for data acquisition and processing. Calibration curves were constructed using the respective analyte/internal standard peak area ratios against analyte concentrations with a weighted (1/C2 ) least-squares linear regression.
Recovery evaluation: The recovery of an analyte was evaluated at three different concentration levels (low, medium, and high-quality controls). For each concentration level, the mean analyte response of six quality-control replicates was compared with that of six extracted control plasma spiked with an appropriate amount of the analyte. The recovery of an internal standard was determined in a similar way except that it was evaluated at only one concentration level and the mean internal standard response was from 18 samples, instead of 6, because the same internal standard concentration was used for all quality control samples.
Matrix selectivity and matrix effect:Matrix selectivity was evaluated by processing 10 randomly selected control human EDTA K2 or EDTA K3 (for sumatriptan only) plasma lots without adding internal standards. A control human plasma lot is considered not interfering with the determination if its responses at or near the retention times of the analyte and its internal standard are less than or equal to 20% and 5% of the mean analyte and internal standard responses of six lower limit of quantitation (LLOQ) samples, respectively.
Matrix effect can be further divided into "absolute" and "relative" matrix effect (8). For quantitative bioanalysis, relative matrix effect is the primary concern. Therefore, only relative matrix effect was evaluated in this article. A total of 10 randomly selected analyte and internal standard–free control human EDTA K2 or EDTA K3 (for sumatriptan only) plasma lots were spiked individually at a low quality-control (QC1) level. Then, three replicates from each lot were analyzed by following sample-processing procedures.
Postpreparative (autosampler) stability: A total of 12 replicates each of the low- and high-quality control (QC1 and QC3) samples were extracted with a calibration curve but only six replicates (comparison samples) were injected with the calibration curve. The remaining six replicates (stability samples) were kept at room temperature under ambient laboratory conditions for a specific period of time. Then, these stability samples were injected with a freshly extracted calibration curve. The mean concentration of stability samples was compared with that of the comparison samples for both low and high QC levels.
Log D calculation: The log D values used in this article were determined by using Pallas software, version 3.1 (CompuDrug Internal, Inc., Sedona, Arizona).
Results and Discussion
The following four evaporation-free extraction methods have been validated including interday and intraday precision and accuracy, recovery, matrix selectivity, and relative matrix effect, dilution, hemolysis effect, potential interference from 10 common over-the-counter drugs, and various stabilities (that is, freeze–thaw, bench-top, long-term, autosampler, blood sample collection and handling). However, to emphasize the extraction methods and to avoid being too lengthy, only the most important validation results that are related closely to the extraction method are presented in this article.

Figure 1
Evaporation-free extraction based upon SPE (elution and injection) for the determination of didanosine (2',3'-dideoxyinosine): Didanosine, a reverse transcriptase inhibitor, is effective against HIV. As shown in Figure 1, didanosine is very hydrophilic. Because of its high hydrophilicity, SPE is usually the method of choice for extraction, as LLE often results in less satisfactory recovery. In SPE, cartridges loaded with highly hydrophilic compounds cannot be washed with high organic washing solution. In this case, it was found that only 5% of methanol can be used during the preliminary experiment. However, a high percentage of methanol has to be used to facilitate the subsequent commonly used evaporation step. After the evaporation step, low organic reconstitution solution — that is, mobile phase of 15% methanol — is used to reconstitute the dry extract. The large difference in organic content between the elution (100%) and the reconstitution solution (15%) dictates an extra filtration or high-speed centrifugation step to remove the insoluble coeluted matrix components, which can otherwise clog the LC system, especially the column. The final sample processing procedure based upon a conventional evaporation-based approach is shown in Figure 2a.
Alternatively, the high hydrophilicity of didanosine can be used as an advantage in developing a faster and more reliable evaporation-free extraction method (Figure 2b). Instead of using high organic elution, which elutes both the analyte and a lot of the other more hydrophobic matrix components, a very low organic elution (20% methanol) is used to elute the analyte, which leaves most of the other more hydrophobic components retained on the cartridges. Because four steps in Figure 2a were removed, the evaporation-free method significantly shortened sample-analysis time. For a typical batch of 150 samples, 3–5 h can be saved.
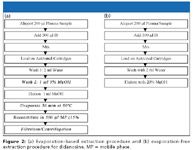
Figure 2
More importantly, much cleaner extracts are obtained in the evaporation-free method. In the evaporation-based extraction, only a very small portion of more hydrophilic matrix components are washed down from the SPE cartridges. Most of the other more hydrophobic matrix components are eluted with the analyte. Though the recovery of analyte might be high, the extracts are not clean, which can cause interferences, ion suppression, and matrix effects. However, a very low organic concentration (20%) was used in the elution for evaporation-free method. Despite this, satisfactory recoveries of 69.04%, 72.83%, and 70.29% were obtained at low, medium, and high quality-control concentration levels, respectively. Furthermore, to evaluate the ruggedness of the extraction, replicates of QC1 were extracted by using three different SPE cartridge lots. The results (Table I) show that not only are the analyte/internal standard peak area ratios comparable, but also are the analyte and internal standard peak areas among different cartridge lots, which demonstrates the good reliability of the method.

Table I: Extraction reproducibility of didanosine usi
Because most of the more hydrophobic matrix components are left on the cartridges, the extracts are much cleaner. With cleaner extracts, there is less possibility of ion suppression. By comparing the areas of properly diluted pure standard solution with those of the extracted control human plasma spiked with the equivalent amounts of analyte and internal standard, no significant ion suppression was observed (1.34% and –3.23% for the analyte and the internal standard, respectively). Despite the slightly lowered recovery in the evaporation-free extraction method, a high signal to noise ratio (S/N) of 178 was obtained at the LLOQ level (25.02 ng/mL). The high S/N and extract cleanliness also can be inferred from the smooth baselines in the representative chromatograms of control human plasma and the well-defined analyte and internal standard peaks in an LLOQ sample (Figure 3).

Figure 3
The removal of evaporation did not impact autosampler stability. The extracted samples can be injected 118 hours after sample processing at room temperature with the percentages of change of 12.54 and 12.11% for low (QC1) and high (QC3) quality controls, respectively. No interference with the analyte and the internal standard nor relative matrix effect were observed from ten randomly selected control human EDTA K2 plasma lots (Table II). As shown in Table III, satisfactory accuracy and precision were obtained. The interday accuracies are within 0.46% difference from the nominal concentrations with CVs≤3.85%. The intraday accuracies are within –4.05% with CVs of 2.27% or less.

Table II: Results of relative matrix effect for didanosine (nominal conc. = 75.36 ng/mL)
The characteristics of this type of evaporation-free extraction method include soft extraction suitable for labile or unstable analytes, as no drastic pH change nor heating is involved; fast and automation-friendly (though it was performed manually in this case, the method can be automated easily using an automatic liquid handling system); very clean extract; and applicability to analytes from hydrophobic to highly hydrophilic. The critical point is to find out the highest possible percentage of organic in washing and the lowest possible percentage of organic in elution. In this way, only the analyte and a few matrix components whose hydrophobicities are close to that of the analyte will be eluted.
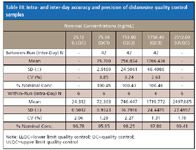
Table III: Intra- and inter-day accuracy and precision of didanosine quality control samples
Evaporation-free extraction based upon SPE (elution, pH adjustment, and injection) for the determination of betamethasone phosphate: Though direct injection of eluate from SPE is desirable as in the didanosine method, it might not be possible in some cases. The elution solution might not be compatible with the mobile phase in terms of pH and buffer composition. In these cases, pH adjustment or introduction of a different buffer is necessary. The method of betamethasone phosphate is such an example.
Betamethasone is an anti-inflammatory drug, which usually is administrated as betamethasone phosphate (Figure 4a) or betamethasone acetate. The phosphate and acetate esters are expected to be hydrolyzed in vivo to betamethasone. In addition, measures should be taken to minimize in vitro hydrolysis (9). As it is quite hydrophilic in neutral and basic media (Figure 4b), an SPE method is preferred for betamethasone phosphate. Because it is charged negatively from acidic to basic conditions, a quick choice would be a mixed-mode anion exchange cartridge. However, the possibility of betamethasone phosphate hydrolyzed to betamethasone would be increased during extraction and evaporation steps owing to heating and significant pH change (strong acid must be used to neutralize the charge of phosphate). Therefore, other types of SPE cartridges are necessary.

Figure 4
In principle, conventional C18 SPE cartridges can be used. Contrary to this conventional approach, mixed-mode strong cation exchange cartridges were chosen, which offers the additional benefit of retaining the unwanted positively charged components on the mixed-mode strong cation exchange cartridges. Furthermore, an evaporation-free approach was integrated to speed up sample cleanup and to further decrease the possibility of conversion during evaporation — that is, heating. The aqueous and the organic washings were performed in weak acidic conditions (2% formic acid), in which betamethasone phosphate is more hydrophobic to avoid analyte loss. After the washings, the analyte was eluted with 50% methanol in near neutral condition, in which betamethasone phosphate is more hydrophilic to increase recovery. As the mobile phase must be weak acidic to retain the analyte long enough on a C18 column, a postelution pH adjustment was necessary. As shown in Figure 5b, 4% formic acid solution was added to the collection tubes before elution. After the analyte was eluted from the SPE cartridges, it was acidified immediately to be compatible in pH with the composition of the mobile phase.
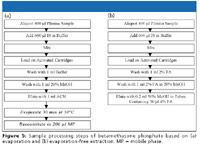
Figure 5
Two steps were removed from the procedures based upon evaporation (Figure 5a), which could save 2–3 h for a typical batch. In addition, the extracts obtained by an evaporation-free approach are cleaner than those from an evaporation-based method because less organic was used in the elution by evaporation-free method.
Despite low elution volume, low organic eluent, and the usage of mixed-mode strong cation exchange (instead of C18 or mixed-mode anion exchange) cartridges, a good S/N ratio of 11 was obtained at the LLOQ level (2.51 ng/mL). The good S/N and extract cleanliness also are shown in the representative chromatograms of control human EDTA K2 plasma and LLOQ samples (Figure 6).

Figure 6
It should be mentioned that although the final extract contains formic acid, its concentration is low (only 0.8%). At this mild acidic condition, betamethasone phosphate is found to be stable for 75 h at room temperature after extraction with the percentages of change of 0.82% and 1.65% for low (QC1) and high (QC3) quality controls, respectively. The selectivity of the method is demonstrated by lack of interference in 10 randomly tested control human EDTA K2 plasma lots and lack of relative matrix effect (Table IV). As shown in Table V, the accuracy and precision of this method are satisfactory.

Table IV: Results of relative matrix effect for betamethasone phosphate (nominal conc. = 7.54 ng/mL)
The method has been applied successfully to two bioavailability studies with a total of 1242 incurred samples from 35 subjects. Only one out of 20 batches did not meet the acceptance criteria. The sample reassay rate was as low as 1.3%, which shows the robustness of the method.
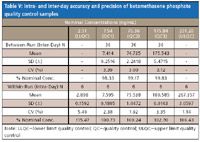
Table V: Intra- and inter-day accuracy and precision of betamethasone phosphate quality control samples
To summarize, this type of evaporation-free method is characteristic of clean extracts, fast and automation-friendly, soft extraction suitable for labile or unstable analytes. In principle, this approach also is applicable for postelution derivatization, dilution, or introduction of buffer or stabilizing agent, which would otherwise be difficult to be mixed in the elution solution.
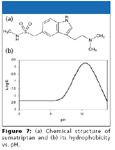
Figure 7
Evaporation-free extraction based upon SPE (high organic wash, low organic elution, direct injection) for the determination of sumatriptan: Sumatriptan, an antimigraine drug, is also very hydrophilic like didanosine (Figure 7). So the dilemma of low organic washing-high organic elution-low organic reconstitution exists for sumatriptan as well. A tedious conventional approach is shown in Figure 8a. However, by studying its hydrophobicity change versus pH (Figure 7b), it was observed that sumatriptan can be more hydrophobic in basic media than in neutral or acidic media. Based upon this characteristic, an evaporation-free extraction method (Figure 8b) was proposed by washing the SPE cartridge with 50% methanol in weak basic pH and elution with 20% methanol in weak acidic pH and injecting the eluate without evaporation. Because the organic solvent concentration in the washing is even higher than that of the elution, extremely clean extract is obtained.
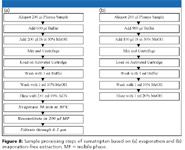
Figure 8
Sumatriptan has shown autosampler stability for at least 50 h at room temperature with the percentages of change of –4.58 and –4.12% for low (QC1) and high (QC3) quality controls, respectively. No interference was observed in matrix selectivity test for 10 randomly selected control human EDKA K3 plasma lots. As shown in Table VI, no significant relative matrix effect was found (only one out of the three replicates for matrix 04(F) showed more than –15% of difference from the nominal concentration, which might be due to sample processing error, however the average of this matrix lot is still within the acceptance criteria).

Table VI: Results of relative matrix effect for sumatriptan (nominal conc. = 3.01 ng/mL)
This evaporation-free extraction method greatly improved the performance of a previously validated method based upon conventional SPE approach in terms of throughput, reliability, sensitivity, and cost (refer to our previously published paper [2] for other validation and application results).
In addition to features such as soft extraction suitable for labile or unstable analytes, speed, and compatibility with automation, the cleanest extract is obtained in this type of evaporation-free extraction. As the organic in the elution is even much lower than that in the preceding washing solution, only few matrix components that are very similar to the analyte can be eluted. This type is most applicable to analytes with variable hydrophobicity. The critical issue is to find a condition where analyte is most hydrophobic and to wash the cartridges with a high percentage of organic solvent in this condition. Then, elute the analyte with a low percentage of organic solvent after adjusting to a different condition at which the analyte is most hydrophilic.
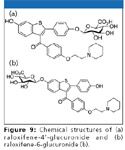
Figure 9
Evaporation-free extraction based upon protein precipitation for the determination of raloxifene-4' and 6-glucuronides: Raloxifene is an antiestrogen used for the treatment of osteoporosis. It undergoes extensive glucuronidation primarily at the 4' and 6-positions of raloxifene (Figure 9). The determination of raloxifene-4' and 6-glucuronides is therefore important in clinical and pharmaceutical research. Though there is a basic functionality in the compounds and mixed-mode strong cation exchange extraction is an easy match, the possibility of the glucuronide conjugates back converted to raloxifene is high due to the drastic pH changes and heating involved in mixed-mode strong cation exchange extraction. A soft and gentle extraction approach is therefore highly desirable to avoid or reduce possible conversion.
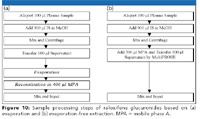
Figure 10
To this end, an evaporation-free extraction method based upon protein precipitation and subsequent dilution of the supernatant was proposed (Figure 10b). To avoid possible evaporation of the transferred supernatant in collection plate while the remaining samples being transferred, 300 µL of low organic mobile phase A was added to the wells in the collection plates before the transfer of supernatant. Because there was no drastic change in pH as in a mixed-mode strong cation exchange method and no heating, no significant conversion was therefore observed in this method. As the evaporation and the subsequent reconstitution steps are removed (refer to Figure 10a), the evaporation-free extraction method is extremely efficient. Two batches with 192 samples/batch can be extracted easily by one lab technician in one shift.

Figure 11
Due to the simple extraction procedure used, high recoveries (over 90%) were obtained for both raloxifene glucuronides. The S/N values at the respective lower limits of quantitation (2.02 and 0.40 ng/mL) are 151 and 81 for raloxifene-4'-glucuronide and raloxifene-6-glucuronide, respectively. The representative chromatograms for control human EDTA K2 plasma and LLOQ samples are shown in Figure 11 and Figure 12. Though both analytes share the same multiple reaction monitoring (MRM) transition, cross-talk or interference is avoided through chromatographic separation.

Figure 12
Similar to other evaporation-free methods described previously, no significant impact on autosampler stability was observed by removing evaporation. Raloxifene-4´-glucuronide is found to be stable for 124 h at room temperature after sample processing with the percentages of change of –12.82 and –13.73% for low (QC1) and high (QC3) quality controls, respectively. Raloxifene-6-glucuronide is found to be stable for 40 h at room temperature after sample processing with the percentages of change of –4.85 and –6.18% for low (QC1) and high (QC3) quality controls, respectively. No interference with the analytes and the internal standards was found in 10 randomly selected control human EDTA K2 plasma lots. As shown in Table VII and Table VIII, there is negligible relative matrix effect for both analytes. The accuracies and precisions of the method are very satisfactory (Table IX and Table X).

Table VII: Results of relative matrix effect for raloxifene-4 (nominal conc. = 6.11 ng/mL)
When this evaporation-free extraction method was applied to the analysis of 2959 incurred samples (a total of 59 subjects), the reassay rates excluding diluted samples were as low as 0.68% and 1.2%, respectively. These results show that the method is accurate, reproducible, and rugged.

Table VIII: Results of relative matrix effect for raloxifene-6-glucuronide (nominal conc. = 1.20 ng/mL)
This type of evaporation-free extraction is extremely efficient and automation-friendly. Probably, it is the softest evaporation-free extraction method. It is applicable to analytes that have high detection sensitivity and high LLOQ values.
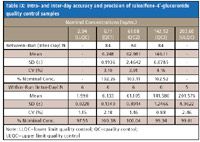
Table IX: Intra- and inter-day accuracy and precision of raloxifene-4 -glucuronide quality control samples
Concentration versus sensitivity or S/N: In addition to being used to evaporation-based extraction approaches, the other main reason why evaporation-free extraction was not widely employed is that the possibility that concentrating an analyte might be lost (as perceived by many). In evaporation-based extraction, the analyte can be concentrated easily by using high sample volume and low reconstitution volume. However, a concentrated extract might not be the analyst's goal; instead, it is the sensitivity or S/N. In other words, a higher concentration might not always lead to high S/N values and high sensitivity, particularly in an MS-based method. As shown in the case of sumatriptan (2), there was no dilution in the original evaporation-based method (sample volume = reconstitution volume = 0.2 mL). The peak height obtained for the LLOQ was about 2100 cps. For the evaporation-free method, there was a fivefold dilution — that is, the analyte in 0.2 mL of sample was eluted by 1 mL of mobile phase. The peak height for the LLOQ in the evaporation-free extraction method was about 6000 cps. The S/N of the evaporation-free method was even higher that of the evaporation-based method despite the dilution factor of 5 (refer to reference 2 for details).
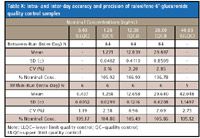
Table X: Intra- and inter-day accuracy and precision of raloxifene-6 -glucuronide quality control samples
The final signal is a product of many factors, such as sample volume, analyte concentration, reconstitution volume, recovery, ion-suppression, and interferences from co-extracted matrix components. Though obtaining concentrated extract can be difficult in some evaporation-free extraction methods, the S/N could be still maintained or even improved through the attainment of cleaner extracts and higher recovery. In addition, cartridges of smaller size can be used to reduce the elution volume, thus, avoiding dilution. Furthermore, as the extracts are cleaner, bigger injection volume can be used to improve S/N.
Conclusions
Developing evaporation-free extraction methods does not simply mean the removal of the evaporation step per se. In many cases, different extraction strategies or approaches are required. The benefits of an evaporation-free extraction method can include reduced pollution and cost; increased method speed; less possibility for contamination and conversion; cleaner extract and improved sensitivity and selectivity in some cases; and better overall reliability.
It is concluded that the evaporation-free methods proposed in this article can be employed both manually and automatically, and are generally applicable in many similar situations as outlined in this article.
Acknowledgments
The authors would like to thank Jean Couture, Brigitte Pellerin, Harshvardhan Patel, Jun Li, Peter Hang, and Nuno Santos for their involvement in some of the work presented in this article.
Aimin Tan*, Saleh Hussain,* and François Vallée†
*Anapharm, Richmond Hill, Ontario, Canada;† Anapharm, Ste-Foy, Québec, Canada
Please direct correspondence to atan@anapharm.com.
References
(1) D. Dell, Chromatographia Supplement 59, S139 (2004).
(2) A. Tan, P. Hang, J. Couture, S. Hussain, and V. Vallée, J. Chromatogr., B 856, 9 (2007).
(3) K. Matsui, Y. Oda, H. Nakata, and T. Yoshimura, J. Chromatogr., B 729, 147 (1999).
(4) H. Zackari and G. Jerndal, paper presented at the 54th ASMS Conference on Mass Spectrometry and Allied Topics, Seattle, Washington, May 28–June 1, 2006.
(5) Y.J. Xue, J. Liu, and S. Unger, J. Pharmaceutical and Biomedical Analysis 41, 979 (2006).
(6) N. Zheng, J. Zeng, Z. Ouyang, S. Pasasfarmer, D.M. Parker, A. Buzescu, J. Lute, M. Jemal, M.E. Arnold, and S.E. Unger, paper presented at the 54th ASMS Conference on Mass Spectrometry and Allied Topics, Seattle, Washington, May 28–June 1, 2006.
(7) A.C. Sanchez, M.M. Kansal, A. Dixon, and I. Rustamov, paper presented at the 31st International Symposium on High Performance Liquid Phase Separations and Related Techniques (HPLC 2007), Ghent, Belgium, June 17–21, 2007.
(8) B.K. Matuszewski, J. Chromatogr., B 830, 293 (2006).
(9) M.C. Petersen, R.L. Nation, W.G. McBride, J.J. Ashley, and R.G. Moore, Eur. J. Clin. Pharmacol. 25, 643 (1983).

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.