Evaluating Particle Size and Back Pressure in Peptide Mapping by UHPLC
Ultrahigh-pressure liquid chromatography (UHPLC) columns are used in peptide mapping to improve the resolution of highly complex peptide mixtures. It is commonly assumed that small particle columns increase performance, but this is not always the case. This study presents a comparison of peak count, column length, and resolution between core–shell and fully porous UHPLC columns.
Ultrahigh-pressure liquid chromatography (UHPLC) columns are used in peptide mapping to improve the resolution of highly complex peptide mixtures. It is commonly assumed that small particle columns increase performance, but this is not always the case. Under some conditions, when factoring in maximum back pressure, small particle columns may not provide the maximum peak capacity or fastest method. This study presents a comparison of peak count, column length, and resolution between core–shell and fully porous UHPLC columns.
Ultrahigh-pressure liquid chromatography (UHPLC) increases the efficiency of LC by the use of sub-2-µm particle media and UHPLC systems with low dead volume and 1000 bar capabilities. UHPLC has been mostly used to reduce run times of existing methods and mobile phase consumption, and has been steadily adopted by the industry. Industry is now split between methods and systems that are 1000 bar compatible (UHPLC) and 400 bar compatible (high performance liquid chromatography [HPLC]).
(PHOTO CREDIT: ANDREW PATERSON/GETTY IMAGES)
The line between UHPLC and HPLC methods has blurred with the introduction of "ultra-high efficiency" core–shell columns that are compatible with traditional 400 bar HPLC systems and that can in some cases provide equivalent, or better, performance than fully porous sub-2-µm columns at low back pressure. This approach has been further complicated by the introduction of sub-2-µm core–shell columns, as well as larger particle columns such as 5-µm core–shell columns (often used in a preparative setting). In most cases, core–shell columns demonstrate improved efficiency over fully porous media of the same particle size.1,2
Peptide mapping is used to determine the amino acid sequence of large proteins (or peptides) that are not amenable to direct characterization by LC coupled to mass spectrometry (MS). Peptides are digested with a sequence-specific proteolytic enzyme (often trypsin) to generate a mixture of smaller peptides that can be run on an LC–MS system.
Small proteins may generate "simple" peptide maps that can be easily separated by reversed-phase HPLC, but when mapping larger proteins, the complexity of the peptide mixture can make identification of individual peptides more difficult; for example, Immunoglobin Ig-G is 150 kilodalton in size and generates a complex mixture of peptides. Longer HPLC gradients can be used by protein chromatographers to increase resolution.
With the advent of UHPLC, many methods have not decreased in run time, instead they have focused on using the increased efficiency of smaller or core–shell particles to improve peptide resolution.
In this article, studies were performed using peptides and peptide maps to compare fully porous and core–shell sub-2-µm UHPLC to larger particle (3.6-µm) core–shell columns under moderate and high linear velocity flow rates, often nearing the back pressure limits of UHPLC columns for both simple and complex mixtures.
Materials and Methods
All reagents were obtained from Sigma Chemical including peptides and proteins, as well as sequencing grade proteolytic enzymes and mobile phase additives. Acetonitrile and water were purchased from EMD Chemical. Peptide mapping specific core–shell columns (Aeris Peptide, Phenomenex) of various particle sizes were compared to commercially available fully porous C18 bonded silica columns (see figures legend for dimensions used). Protein digests were generated by incubating proteins for 12 h at 37 °C after inoculation with sequencing grade trypsin (1:50 E/S ration). The sample was quenched with formic acid prior to injection.
Peptide standards and digests were run on an Acquity I-class UHPLC system (Waters)using Empower software (Waters). Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile. For the peptide separation on 50 mm length columns, the flow rate was either 0.3 mL/min or 0.9 mL/min with a gradient from 5% to 65%B in either 10 min or 1.26 min, respectively. For the protein digest, the flow rate was 0.5 mL/min with a gradient from 3 to 65%B in 15 min. Columns were maintained at 50 °C and peptide elution was monitored at 214 nm. Any deviations to such conditions are listed in the figure legends.
Results and Discussion
Figure 1 shows the results for a simple mixture of peptides run on the 1.7-µm core–shell and fully porous columns, as well as the 3.6-µm core–shell columns. When compared at moderate flow rates and with a simple mixture of peptides, all three columns demonstrated similar resolution, with the core–shell columns demonstrating slightly narrower peak widths than the fully porous sub-2-µm column. While both 1.7 µm columns operated at ~270 bar (well within HPLC back pressures), the 3.6-µm core–shell column demonstrated equal performance at around one third (~87 bar) the back pressure of the sub-2-µm columns.

Figure 1: Resolution example for a mixture of peptides (Sample: 1. bradykinin; 2. dynorphin A; 3. angiotensin II; 4. met encephalin; 5. leu encephalin) at moderate linear velocities run on 50 à 2.1 mm columns. Note the improved resolution and peak width for the coreâshell columns over fully porous columns. The 3.6-µm coreâshell column has significantly lower back pressure amenable to HPLC with similar performance to the sub-2-µm columns.
When very high linear velocities (0.9 mL/min) were used, a similar back pressure ratio was observed (Figure 2); the 3.6-µm core–shell still operated at HPLC back pressure while both the 1.7-µm columns (core–shell and fully porous) operated at pressures only compatible with a UHPLC system. When one looks closely at the chromatography, all three columns have peak widths on a par with each other (the sub-2-µm core–shell column demonstrated >10% reduction in peak width over the fully porous column); however, the 3.6-µm core–shell did lose significant resolution over the early eluting peptides, which could be minimized with some gradient optimization or it might be inherent with the increased eddy diffusion distances of the larger particle core–shell media.

Figure 2: Resolution example for a mixture of peptides (Sample: 1. bradykinin; 2. dynorphin A; 3. angiotensin II; 4. met encephalin; 5. leu encephalin) at high linear velocities run on the same 50 à 2.1 mm columns. Note the improved resolution and peak width for the 1.7-µm coreâshell columns over fully porous columns. The 3.6-µm coreâshell column still has HPLC compatible back pressure; however, the resolution of peak 1 and 2 has been compromised.
The first experiment looked at simple mixtures using shorter UHPLC columns, where resolution might not be an issue. In the second experiment, peptide digests of apo-myoglobin were analyzed on longer 150 × 2.1 mm columns where increased resolution might be required in a separation. The flow rate was set at 0.5 mL/min, which is at the maximum pressure limit (~1000 bar) for the sub-2-µm core–shell and fully porous columns (Figure 3). Under equivalent conditions the 3.6-µm core–shell column had similar peak counts with back pressures (350 bar) still amenable to standard HPLC use (albeit with wider peak widths than the sub-2-µm core–shell column). This lower column back pressure could be utilized to reduce run time with increased mobile phase velocity by raising the flow rate to 0.9 mL/min and reducing the gradient time to 7.5 min. Alternatively, a longer column (250 × 2.1 mm) could be used with a slightly longer gradient (10 min) to increase resolution while still acquiring reduced peak widths and shorter run times than the sub2-µm core–shell and fully porous columns (Figure 4).
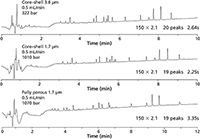
Figure 3: Example of an Apo-myoglobin digest run on 150 à 2.1mm columns. Note that the sub-2-µm columns are at their back pressure limits while the 3.6-µm coreâshell column produces a similar performance at HPLC compatible back pressures.
Summary
With the advent of sub-2-µm columns and UHPLC systems capable of operating at 1000 bar back pressure, the trend has been to use shorter columns with smaller particles to get faster high-resolution methods. In the case of small molecule separations, especially when run isocratically or in gradient mode with ballistic gradients, the use of short columns can provide a fast solution with equivalent or better resolution than traditional HPLC systems. However, with core–shell columns of various particle sizes the need for ultra-high pressure is not always required. As has been shown previously, core–shell columns can give higher efficiency than fully porous columns of the same particle size. This means it is possible to get a core–shell column with a particle size greater than 2 µm to be used to get equivalent or better results of fully porous sub-2 µm column at back pressure amenable to standard HPLC.3
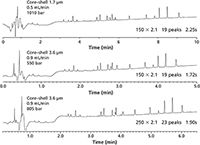
Figure 4: Higher flow rates and faster gradients are used to further reduce run times for the 3.6-µm coreâshell peptide column while achieving improved peak width under UHPLC conditions. Switching to longer columns (250 à 2.1 mm) can further increase resolution even with shorter run times than the sub-2-µm columns.
With peptide mapping applications the focus is typically on improved resolution over speed and therefore longer columns are used to increase total plate counts for separation (versus plates per metre) to maximize resolution and increase peak counts of complex separations. Once columns get to a length of 150 mm or longer, using sub-2-µm media columns (either core–shell or fully porous media) starts to become a liability because one is now approaching the back pressure limits of 1000 bar UHPLC systems. Because the pressure drop for larger particle core–shell materials is lower, HPLC systems can be used for some of the same separations.
However, in many cases core–shell columns with particles larger than 2 µm have operating limits similar to sub-2-µm core–shell columns and therefore can be used at higher linear velocities than sub-2-µm materials. In peptide mapping applications, where resolution is the key focus of separations, the longer column with more absolute plates will usually be the better solution for maximum resolution. In many cases the larger particle with the lower pressure drop will potentially be the better solution for going faster under UHPLC conditions because of the back pressure limitations that sub-2-µm media (both core–shell or fully porous) may place on separation at high linear velocities.
References
1. S.Fekete et al., Journal of Pharmaceutical Research and Biomedical Analysis 54(3), 482–490 (2011).
2. K. Broeckhoven et al., Journal of Pharmaceutical Analysis 3(5), 313–323 (2013).
3. Fekete et al., Journal of Chromatography A 1236, 177–188 (2012).
Michael McGinley is a senior product manager at Phenomenex and focuses on the development and management of Phenomenex's bioseparation portfolio. He brings over 25 years of expertise in chromatography and biopharma drug development. He is a well published contributor and a member of LCGC's editorial advisory board.
Jason Anspach is a senior scientist at Phenomenex and is responsible for managing the team engineering next generation separation products. Also well published, Jason's current focus is on pushing the performance boundaries of core–shell column technologies.
E-mail: michaelm@phenomenex.com
Website: www.phenomenex.com
This article is from The Column. The full issue can be found here:http://images2.advanstar.com/PixelMags/lctc/digitaledition/September08-2014-uk.html#2

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.












