Enhancing PLGA Characterization with Multi-Angle Light Scattering and Differential Viscometry
The functional properties of polymers, such as poly(lactic-co-glycolic acid) (PLGA), relevant to drug delivery and biomedical devices, are governed by the molecular properties of molar mass, composition, conformation, and branching. This article demonstrates how such polymers are fully characterized, quickly and absolutely, using gel permeation chromatography (GPC) with multi-angle light scattering (MALS) and online viscometry.
Photo Credit: LoritaR/Shutterstock.com

The functional properties of polymers, such as poly(lactic-co-glycolic acid) (PLGA), relevant to drug delivery and biomedical devices, are governed by the molecular properties of molar mass, composition, conformation, and branching. This article demonstrates how such polymers are fully characterized, quickly and absolutely, using gel permeation chromatography (GPC) with multi-angle light scattering (MALS) and online viscometry.
Poly(lactic-co-glycolic acid) (PLGA) is a widely used polymer for drug delivery and biomedical devices, including sutures and prosthetic devices, because of its biocompatibility and biodegradability. As a drug delivery vehicle, PLGA extends the release of its payload to reduce dosing frequency, and as of 2016, there were 15 FDA-approved PLGA- or PLA-based drug products available in the US (1,2).
The drug-release profile is controlled by the overall molar mass and ratio of lactide to glycolide (L:G ratio). For example, drug delivery vehicles commonly make use of 50:50 L:G ratios to control the drug release profile (3–5). PLGA with higher L:G ratios tend to degrade more slowly because of the hydrophobic character of the lactide side chain (2). Therefore, characterization of L:G ratio and structure is crucial for developing safe and effective therapeutics. This article describes the characterization of PLGA samples with distinct L:G ratios using gel permeation chromatography (GPC) coupled with multi-angle light scattering (MALS) and intrinsic viscosity (IV) measurements. Application of GPC-MALS-IV includes identification and characterization of PLGA samples with unknown L:G ratios, quality control of different lots of material, and comparison among manufacturers.
In a GPC-MALS-IV method, MALS directly measures the molar mass of the eluting polymer, regardless of elution time and without relying on molar mass standards; differential viscometry enables characterization of the polymer’s intrinsic viscosity, hydrodynamic size, and conformation. The combination of MALS and IV provides the widest measurement range and highest sensitivity, quantifying molar masses from a few hundred g/mol up to 109 g/mol and radii below 1 nm up to ~1 µm. The relationship between intrinsic viscosity [η] and molar mass is often displayed using a Mark-Houwink–Sakurada (MHS) plot, whose slope and intercept are used to determine branching parameters. Linear polymers in thermodynamically good solvents typically exhibit MHS slopes around 0.5, and extended conformations can increase the slope up to a value of 1 for rod-like structures.
Branching increases the polymer density because monomer units are added to the branches and not just the ends of the monomer. The result is a polymer with higher molar mass at the same overall Rg or [η] as its linear analogue. Polymers with long chain branching (LCB) exhibit a lower slope in an MHS plot than their linear analogues. Short chain branching (SCB) also creates a dense structure with lower intrinsic viscosity than a linear polymer of the same molecular weight. However, the overall conformation (MHS slope) remains similar to the linear analogue because of the short branch lengths; thus, SCB phenomena are visualized as parallel MHS plots. For PLGA, conformational analysis using MHS plots clearly differentiates among the L:G ratios tested and may help elucidate their functionality.
Methods
PLGA standards with varying L:G ratios (Sigma) were analyzed via GPC-MALS-IV. The separation was performed using an Agilent 1100 autosampler and two 7.5 mm × 300 mm, 5-µm PLgel mixed-C columns (Agilent) with THF as the mobile phase at a flow rate of 1 mL/min. Samples were prepared at 5 mg/mL in THF, and 100 µL of each sample was injected onto the column. The effluent from the columns flowed through a DAWN multi-angle light scattering detector, ViscoStar differential viscometer, and Optilab differential refractometer (Wyatt Technology Corporation). The light scattering, concentration, and viscometry data were collected and analyzed using ASTRA software (Wyatt). A constant refractive index increment (dn/dc) of 0.049 mL/g was used for the analysis of all PLGA samples.
One major advantage of incorporating MALS and IV with GPC is that the measurement is independent of elution time. This is critical for PLGA analysis because the polymer may not share the same conformation as a “typical” molecular weight standard.
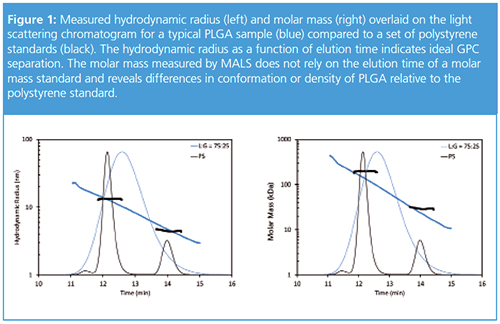
Figure 1 shows a typical chromatogram for PLGA overlaid with data for polystyrene standards. At each eluting slice, the molar mass, concentration, and intrinsic viscosity are measured directly, and the hydrodynamic radius is calculated from the intrinsic viscosity. The measured hydrodynamic radius vs. elution time indicates the separation is ideal: the PLGA is eluting according to its hydrodynamic volume and is not interacting with the column (Figure 1, left). However, estimating the molar mass of PLGA based on its hydrodynamic volume (or elution time) would significantly overestimate the molar mass of the PLGA (Figure 1, right). Clearly, the PLGA is less dense than the polystyrene standard since molecules of the same hydrodynamic size have a lower molar mass relative to polystyrene.
Results
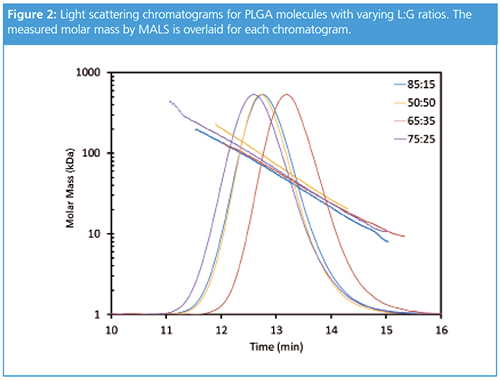
The GPC-MALS-IV data provided key insights into the molar mass, size, and conformation of the different PLGA molecules. Figure 2 shows typical chromatograms for PLGA molecules with four different L:G ratios, and the molar mass moments are summarized in Table 1. Although the 75:25 LG ratio elutes first, its molar mass is actually less than that of the 50:50 PLGA, which elutes second. Moreover, despite nearly identical elution profiles, the molar mass measured by MALS for the 50:50 LG ratio is 30% greater than that measured of the 85:15 LG ratio. This mismatch in measured molar mass and elution volume points to differences in density and conformation among the different types of PLGA.
Intrinsic viscosity measurements provide additional detail regarding polymer size and conformation. Although all of the PLGA molecules were linear, they exhibited different conformations based on their L:G ratios. The MHS plot in Figure 3 highlights key differences for each type of PLGA.
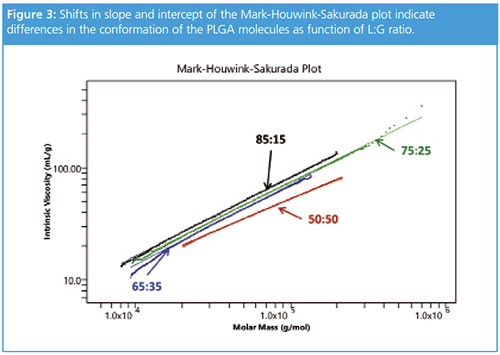
First, with increasing glycolic acid content, the PLGA becomes more dense. Thus, for a given molar mass, a 50:50 L:G ratio produces the most compact conformation and smallest hydrodynamic size of all the L:G ratios tested. Second, the slope and intercept of the MHS plot conveys additional information about the molecular conformation. The slope of 0.57 for the 50:50 PLGA suggests a random coil, while the slope of ~0.7 for the other three samples suggests a more extended conformation.
Despite the nearly identical slopes for the 65:35, 75:25, and 85:15 polymers, the conformation data do not overlay. Instead, they form parallel lines in the MHS plot. The parallel conformation plots are often indicative of short-chain branching, but in this case the differences among the molecules are more subtle. Since these PLGA controls are unbranched, the increase in density at higher L:G ratios is caused by the extra methyl side chain in the lactide monomer, which is not present in the glycolide monomer. This difference, although small, produced parallel MHS plots with good reproducibility across multiple sample injections, highlighting the robustness of the GPC-MALS-IV measurement.
Conclusions
Combining MALS and differential viscometry with GPC enables direct measurement of the molar mass and intrinsic viscosity of PLGA samples and highlights significant differences in conformation among the different L:G ratios. Even though all the tested samples were linear, each L:G ratio produced a distinct set of Mark–Houwink parameters and distinct elution profile.
Importantly, these differences would not be evident by analytical GPC alone. GPCâMALSâIV characterization can be further extended to classify unknown PLGA samples based on known standard conformation and also to evaluate branching and other macroscopic structural differences.
References
- Y. Wang, W. Qu, and S.H. Choi, American Pharmaceutical Review19(4), 5–9 (2016).
- M. Allahyari and E. Mohit, Human Vaccines & Immunotherapeutics12, 806–828 (2016).
- M. Marimuthu, D. Bennet, and S. Kim, Polymer Journal 45, 202–209 (2013).
- Y.-Y. Tseng, Y.-C. Wang, C.-H. Su, and S.-J. Liu, Scientific Reports5, 7849 (2015).
- S.W.N. Ueng et al., J. Orthop. Surg. Res.11, 52 (2016).
Sophia Kenrick is senior applications scientist at Wyatt Technology where she supports multiple applications for Wyatt instrumentation, especially in the field of molecular recognition and biomolecular interactions. Sophia received a bachelor’s degree in chemical engineering from Arizona State University (Arizona, USA), a doctorate in chemical engineering from the University of California, Santa Barbara (USA), and has been with Wyatt since 2010. She is also the Dean of Light Scattering University, Santa Barbara campus.

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













