Combining Sorptive Extraction with Two-Dimensional Gas Chromatography for the Flavour Profiling of Milk
The Column
This proof-of-principle study shows that polymer-based sorptive extraction probes, coupled with secondary focusing by thermal desorption and analysis by flow-modulated GC×GC–TOF-MS/FID, can be used to separate and identify flavour compounds in milk. As well as comparing the profiles of dairy and non-dairy milks, this article highlights the practical benefits of this sampling procedure, the ability of two-dimensional GC to physically separate components that would coelute in one-dimensional GC, and the use of software tools to improve workflow.
Photo Credit: Steve80/Shutterstock.com

This proof-of-principle study shows that polymer-based sorptive extraction probes, coupled with secondary focusing by thermal desorption and analysis by flow-modulated two-dimensional gas chromatography with time-of-flight mass spectrometry or flame ionization detection (GC×GC–TOF-MS/FID), can be used to separate and identify flavour compounds in milk. As well as comparing the profiles of dairy and non-dairy milks, this article highlights the practical benefits of this sampling procedure, the ability of two-dimensional GC to physically separate components that would coelute in one-dimensional GC, and the use of software tools to improve workflow.
Like many other foodstuffs and beverages, the quality of milk as perceived by the consumer depends crucially on the presence of aroma-active compounds. This sensitivity can be attributed in part to the relatively bland flavour of milk, which allows even modest changes in compound profiles to be perceived (1). The flavour of milk is nevertheless influenced by the presence of a large number of compounds from numerous chemical groups, many present at levels below their flavour threshold (2). Reliable analysis of the volatile components of milk is therefore valuable in research to understand the factors affecting the flavour profile, and in routine monitoring to ensure high quality or identify the cause of off-flavours.
Approaches to sampling of milk volatiles are numerous (3). In the past, liquid–liquid extraction (LLE), solid-phase extraction (SPE), and distillation were widely used, often in studies that focused on the fatty-acid components of the volatile organic compound (VOC) profile by derivatizing them to their methyl esters. However, in the last 20 years these methods have largely been supplanted by headspace approaches (or less often, purge-and-trap [4]). Headspace analysis provides profiles that are representative of the volatile emissions from the sample, as well as offering greater operational simplicity and reduced artefact levels (5). However, the low concentrations involved means that a preconcentration stage is essential, and to this end sampling of the headspace onto a sorbent is used-typically either in static headspace mode using a solid-phase microextraction (SPME) fibre (6), or using a dynamic headspace device in conjunction with a sorbent-packed tube and transfer to the gas chromatography (GC) system via trap-based thermal desorption (7,8,9).
Despite the advantages and popularity of these approaches, there remains a place for easy-to-use methods able to extract volatiles from the bulk liquid, both for a deeper understanding of the full volatile content of the liquid sample (as opposed to the subset of compounds released into the vapour phase), and for monitoring less-volatile organic compounds such as contaminants. SPME fibres, although occasionally used for immersive sampling of milk (10), are easily broken (for example during the necessary washing stage), offer limited sensitivity, and may also suffer from capillary-action carryover effects. Sampling onto a relatively large volume of monolithic poly(dimethylsiloxane) (PDMS) addresses all these issues, and has been shown to give good results for the sampling of milk in two configurations-fitted around a glass-coated magnetic stir-bar (11), and likewise on a stainless steel probe (12) (used in this study). The analytical performance of both sampling formats is comparable, but the probes are far easier to manipulate, and are also amenable to automation. In both cases the volatiles are desorbed within a thermal desorption tube, followed by trap-based preconcentration. Recent work (13) has shown that probe-based sorptive extraction can extract a similar number of compounds from milk as headspace-SPME, but with an emphasis on lower-volatility compounds with lower polarity (which have a greater affinity for the PDMS sorptive phase).
Irrespective of the sampling approach, analysis of volatiles usually proceeds by GC–mass spectrometry (MS), but as in the case of many foods and beverages, the presence of structurally similar compounds in milk aroma profiles often gives rise to coelution with regular one-dimensional GC configurations. This is usually only tackled by long (but expensive) columns, or by multiple analyses that decrease productivity. To address this, food analysts are increasingly using GC×GC, which allows efficient separation of homologues and isomers, and can enhance analyte capacity by up to an order of magnitude (14).
This study demonstrates an approach to the analysis of volatile compounds in four types of liquid milk, by combining probe-based immersive sorptive extraction with GC×GC. A reverse-fill/flush flow modulator avoids the cost and logistical issues associated with thermal modulators using liquid cryogen (15), and parallel detection by time-of-flight (TOF)-MS and flame ionization detection (FID) enables both easy identification of unknowns and straightforward quantitation. To the best of our knowledge, this is the first reported example of milk analysis by high-capacity sorptive extraction combined with GC×GC.
Experimental
Samples: Whole cow’s milk, goat’s milk, soya milk, and almond milk were purchased from a local supermarket. A 10-mL measure of each sample was placed in a 20-mL headspace vial with 2 g of NaCl, to improve extraction efficiency by decreasing the solubility of analytes.
Immersive Sorptive Extraction: PDMS sampler: Inert HiSorb probe (Markes International); time: 60 min; temperature: 35°C.
Thermal Desorption: Instrument: TD100-xr (Markes International); focusing trap: “General-purpose”. HiSorb probes were inserted into empty inert-coated stainless steel TD tubes.
GC×GC: Flow modulator: Insight (SepSolve Analytical). PM: 5.0 s.
TOF-MS: Instrument: BenchTOF-Select with Tandem Ionization (SepSolve Analytical): Simultaneous acquisition of 70 eV and 12 eV data; mass range: m/z 35–500.
Software: ChromSpace GC×GC software (SepSolve Analytical) for full instrument control and data processing.
Results and Discussion
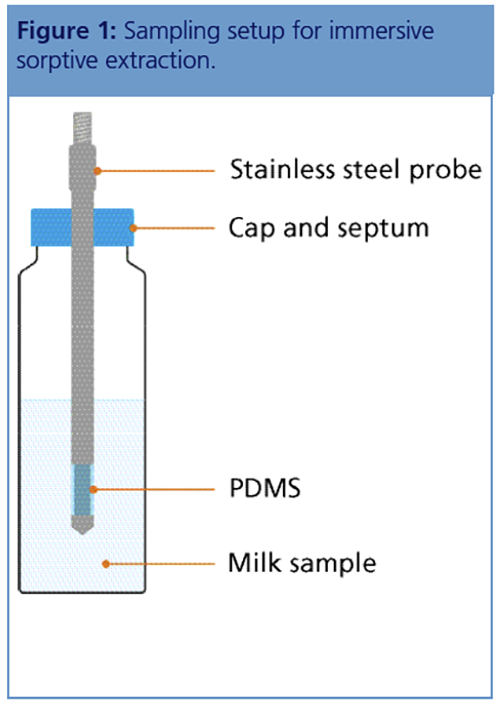
Sampling and Analytical Considerations: The high-capacity sorptive extraction probes used in this study consist of a short section of PDMS located near the end of a stainless-steel probe, which is immersed in the sample (Figure 1). Agitation with gentle heating is then sufficient to ensure that analytes are effectively absorbed into the sorbent volume in a reasonable time frame. A key consideration is the relatively large sorbent volume (65 μL compared to 0.5 μL for SPME), which, combined with secondary focusing by thermal desorption (TD), results in higher sample loadings and therefore greater sensitivity across a wide analyte range.
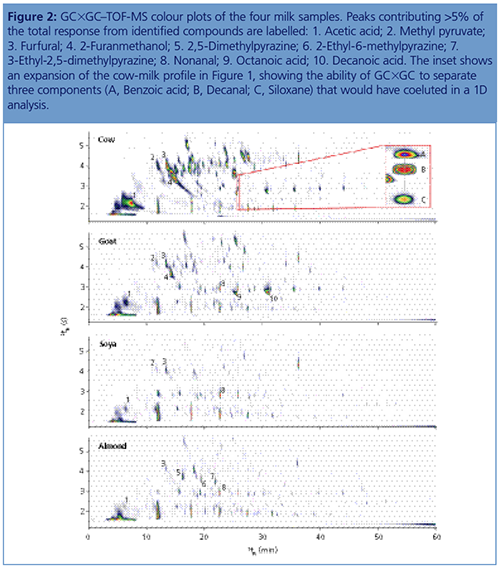
The GC×GC–TOF-MS colour plots obtained from the four milk samples are shown in Figure 2, showing the excellent separation achieved. A common issue with any sampling technique employing polymeric silicone stationary phases is the presence of siloxane contaminants in the chromatogram, but the second separation in GC×GC ensures that the aroma and flavour compounds of interest are well-separated from these (Figure 2, inset).
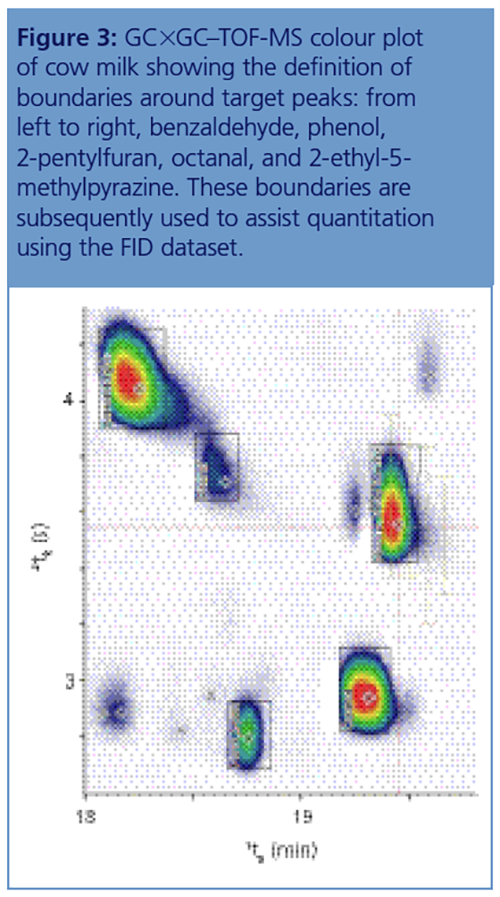
Analysis by TOF-MS is ideal for research or investigations into unknown contaminants, but routine quantitative analysis is best performed using FID, and in this study a dual TOF-MS/FID setup was used. Quantitation using this approach involves first identifying compounds using TOF-MS, with tightly defined boundaries being drawn around the identified peaks (Figure 3). This collection of boundaries, known as a stencil, can then be transferred to the FID dataset, enabling rapid quantitation of all the target peaks.
Sample Comparison: Figure 4 shows the relative abundances for the various chemical classes resulting from the application of this approach to two dairy milks (cow milk, goat milk) and two non-dairy milk substitutes (soya milk, almond milk). A total of 57 compounds were identified across the four samples, with 45, 39, 20, and 19 being found in cow, goat, soya, and almond, respectively-only 10 compounds were present in all samples. As well as this difference in compound diversity, it is immediately apparent from Figure 4 that the cow milk showed a much greater overall response compared to the other samples, which largely derives from greater responses for the acids, furans, ketones, and pyranones. In contrast, the goat milk had a markedly weaker response, and the two non-dairy milk substitutes were weaker still.
Medium-chain fatty acids are important components of dairy products, and decanoic acid was the most abundant of this group in both the cow and goat milks, followed by octanoic acid (linear and branched-chain C8–C10 acids have been noted to impart a distinctive “goat-like” flavour [16]). However, both samples contained a substantial quantity of acetic acid, and in the cow milk this dwarfed the other acids present by a ratio of 3.5 to 1, which may indicate a degree of rancidity.
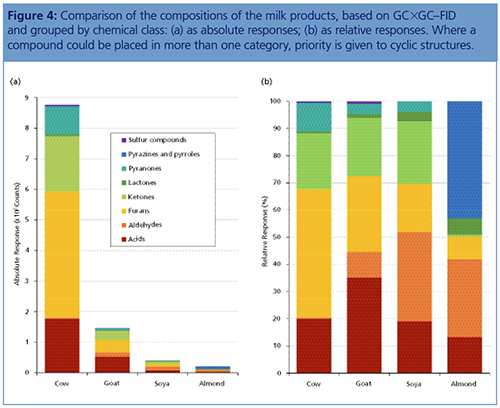
Medium-chain esters have been reported to be predominant odorants in raw milk but of reduced importance in pasteurized milk (17), and in agreement with this no compounds from this group were detected in these two pasteurized samples. However, some cyclic esters were observed, of which the most predominant were δ-decalactone, δ-dodecalactone, and δ-octalactone (“lactonic/sweet/coconut/fruity” [2]). Methyl pyruvate made a significant contribution to the response in the cow’s milk, and may be the result of fermentation (18).
Heterocyclic compounds were a major component of the cow milk sample, with 17 furan derivatives and five pyranones present, including as major components the maltol, isomaltol, and 2-furanmethanol (“burnt/caramellic” [19]); compounds of this group have been found in sterilized concentrated milk (1). Ketones are also important flavour contributors, and in the goat milk heptan-2-one (“blue cheese, spicy” [1,20]) and nonan-2-one (“mustard-like, spicy” [1]) were identified. The latter two compounds are also reportedly responsible for “UHT flavour” (2). Amongst low-boiling sulfur compounds, dimethyl sulfone was found in both the cow and goat milks, in accordance with its identification as a significant odorant in these two products (21).
Compared to the dairy products, the soya milk had a distribution of abundances between the compound classes that is not too dissimilar, albeit with much higher responses for some medium-chain aldehydes. Amongst these, nonanal (“sweet, floral, green, grass-like” [1]) predominated, making an interesting comparison with an earlier study of soya milk headspace (22), which as expected found the more volatile congeners (pentanal and hexanal) to dominate. In contrast, the almond milk showed a quite different profile, with a distinguishing feature being the presence of a range of pyrazines, some of which are known to impart a “toasted almond” flavour (23). One of these is 2,5-dimethylpyrazine, noted to be partly responsible (along with 2-methoxy-3-isopropylpyrazine) for a “musty, potato-like” aroma defect in milk (24).
Conclusions
In this study, it has been shown that combining high-capacity sorptive extraction and GC×GC can enable the characterization of the complex flavour profiles of milk and related products, allowing useful cross-sample comparisons to be made. Compared to SPME, the sampling protocol is more robust for immersive extraction, while retaining the ability to sample a wide range of chemical classes. In addition, the use of flow-modulated GC×GC provides high chromatographic resolving power without the need for liquid cryogen, while software tools streamline data analysis from simultaneously acquired TOF-MS and FID datasets.
References
- I.V. Wolf, C.V. Bergamini, M.C. Perotti, and E.R. Hynes, in Milk and Dairy Products in Human Nutrition: Production, Composition and Health, Y.W. Park and G.F.W. Haenlein, Eds. (John Wiley and Sons, 2013), ch. 15.
- H.T. Badings, in Volatile Compounds in Foods and Beverages, H. Maarse, Ed. (Marcel Dekker, 1991), ch. 4.
- R. Mariaca and J.O. Bosset, Lait 77, 13–40 (1997).
- Y. Naudé, M. van Aardt, and E.R. Rohwera, Journal of Chromatography A1216, 2798–2804 (2009).
- B. Toso, G. Procida, and B. Stefanon, Journal of Dairy Research 69, 569–577 (2002).
- É.A. Souza-Silva, E. Gionfriddo, and J. Pawliszyn, TrAC Trends in Analytical Chemistry71, 236–248 (2015).
- R. Imhof and J.O. Bosset, LWT – Food Science and Technology27, 265–269 (1994).
- T. Jansson, S. Jensen, N. Eggers, M.R. Clausen, L.B. Larsen, C. Ray, A. Sundgren, H.J. Andersen, and H.C. Bertram, Dairy Science & Technology94, 311–325 (2014).
- N. Intawiwat, M.K. Pettersen, E.O. Rukke, M.A. Meier, G. Vogt, A.V. Dahl, J. Skaret, D. Keller, and J.P. Wold, Journal of Dairy Science93, 1372–1382 (2010).
- F. Bianchi, M. Careri, A. Mangia, M. Mattarozzi, and M. Musci, Journal of Chromatography A41–45, 1196–1197 (2008).
- Gerstel Application Note, Determination of flavor and off flavor compounds in dairy products using stir bar sorptive extraction (SBSE) and thermal desorption GC/MSD/PFPD (AppNote 5/2000), Gerstel (2000).
- Markes Application Note, Flavour profiling of milk using HiSorb sorptive extraction and TD–GC–MS (Application Note 120), Markes International (2016).
- H. Faulkner, T.F. O’Callaghan, S. McAuliffe, D. Hennessy, C. Stanton, M.G. O’Sullivan, J.P. Kerry, and K.N. Kilcawley, Journal of Dairy Science101, 1–14 (2018).
- J.B. Phillips and J. Beens, Journal of Chromatography A856, 331–347 (1999).
- J.F. Griffith, W.L. Winniford, K. Sun, R. Edam, and J.C. Luong, Journal of Chromatography A1226, 116–123 (2012).
- F. Morgan and P. Gaborit, International Journal of Dairy Technology54, 38–40 (2001).
- L. Moio, J. Dekimpe, P. Etievant, and F. Addeo, Journal of Dairy Research60, 199–213 (1993).
- J.A. Narvhus, K. Østeraas, T. Mutukumira, and R.K. Abrahamsen, International Journal of Food Microbiology41, 73–80 (1998).
- The Good Scents Company Information System (search facility): www.thegoodscentscompany.com/search2.html (accessed on 8 August 2018).
- L. Moio, P. Etievant, D. Langlois, J. Dekimpe, and F. Addeo, Journal of Dairy Research61, 385–394 (1994).
- L. Moio, D. Langlois, P. Etievant, and F. Addeo, Journal of Dairy Research60, 215–222 (1993).
- A. Achouri, J.I. Boye, and Y. Zamani, Food Chemistry99, 759–766 (2006).
- L. Vázquez-Araújo, A. Verdú, P. Navarro, F. Martínez-Sánchez, and Á.A. Carbonell-Barrachina, International Journal of Food Science & Technology44, 2225–2233 (2009).
- M.E. Morgan, Biotechnology and Bioengineering XVIII, 953–965 (1976).
Rebecca Preston graduated from Teesside University in 2014 with a first-class honours degree in forensic science. Following this, she worked at LGC as a human testing scientist, which involved extracting and analyzing anabolic steroids and new psychoactive substances from human matrices. In early 2017 she joined SepSolve, where she supports internal sales and coordinates the development of applications within the laboratory.
Laura McGregor received an M.Chem. in chemistry from the University of St Andrews, UK, followed by an M.Sc. in forensic science at the University of Strathclyde, UK. Her Ph.D. in environmental forensics, also at the University of Strathclyde, focused on the chemical fingerprinting of environmental contamination using advanced techniques such as GC×GC−TOF-MS. In her current role, she specializes in the application of GC×GC and TOF-MS to challenging applications.
David Barden studied natural sciences at the University of Cambridge, UK, and remained there for his Ph.D. in synthetic organic chemistry, which he received in 2003. A placement at Wiley-VCH, Germany, was then followed by seven years as a journals editor at Royal Society of Chemistry Publishing, UK, before beginning his current role as copywriter in 2011.
E-mail:hello@sepsolve.comWebsite: www.sepsolve.com

Analysis of Pesticides in Foods Using GC–MS/MS: An Interview with José Fernando Huertas-Pérez
December 16th 2024In this LCGC International interview with José Fernando Huertas-Pérez who is a specialist in chemical contaminants analytics and mitigation at the Nestlé Institute for Food Safety and Analytical Sciences at Nestlé Research in Switzerland, In this interview we discuss his recent research work published in Food Chemistry on the subject of a method for quantifying multi-residue pesticides in food matrices using gas chromatography–tandem mass spectrometry (GC–MS/MS) (1).
The Use of SPME and GC×GC in Food Analysis: An Interview with Giorgia Purcaro
December 16th 2024LCGC International sat down with Giorgia Purcaro of the University of Liege to discuss the impact that solid-phase microextraction (SPME) and comprehensive multidimensional gas chromatography (GC×GC) is having on food analysis.










