Enhanced Results of Diesel Range Organics Analysis with High-Temperature Gas Enhanced Chromatography
The Application Notebook
Phenomenex Application Note
Diesel range organic (DRO) compounds have boiling points similar to diesel fuel (C10–C28), ranging from 170–430 °C. These heavy compounds are amenable to GC-FID analysis; however, important considerations should be taken to ensure reproducible results over repeated injections.
While DRO analytical methods may not reach excessively high temperatures, periodic elevated oven ramps should be used to condition the column and remove contaminants. Without a rugged, high-temperature column, the oven ramp may not sufficiently remove contaminants, resulting in decreased reproducibility over repeated injections. To decrease run times, chemists may apply aggressive oven ramps that approach typical columns' temperature limits. Repeated use of a column near its temperature limit can negatively impact its lifetime. For these reasons, high-temperature GC columns are desired for this application. Zebron™ Inferno™ GC columns have the highest temperature limits of any non-metal GC columns (up to 430 °C), and, therefore, provide the flexibility to increase throughput with aggressive oven ramps, extend column lifetime with rugged temperature stability, and improve reproducibility by baking off contaminants at higher temperatures. This study presents a 10min method for analysing DROs using a Zebron ZB-5HT Inferno GC column.
Experimental Conditions
An Agilent 6890 GC/FID was used. Conditions are shown in Table 1.
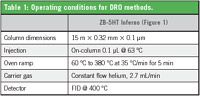
Table 1: Operating conditions for DRO methods.
Results
The resultant chromatogram for this diesel analysis is shown in Figure 1. The on-column injection technique reduced inlet discrimination and increased sensitivity of late-eluting compounds. To further improve sensitivity, a large-volume injection could be employed. To view a 20 µL injection method, visit www.phenomenex.com and search for Application 18153.
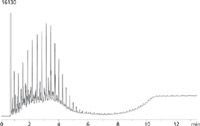
Figure 1: Diesel fuel on ZB-5HT. Sample was 0.1 mg/mL in dichloromethane.
Conclusions
A robust method was presented for DRO fraction analysis using an oncolumn injection technique. A large-volume injection is suggested for labs interested in improved sensitivity, reduced volumes of extracted samples, and cost-savings. The use of high-temperature columns allowed safe conditioning at elevated temperatures for increased reproducibility and column lifetime.
Phenomenex Inc.
411 Madrid Avenue, Torrance, California 90501, USA
tel. +1 (310) 212-0555, +1 (310) 328-7768
Website: www.phenomenex.com

Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Studying Cyclodextrins with UHPLC-MS/MS
May 5th 2025Saba Aslani from the University of Texas at Arlington spoke to LCGC International about a collaborative project with Northwestern University, the University of Hong Kong, and BioTools, Inc., investigating mirror-image cyclodextrins using ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) and vibrational circular dichroism (VCD).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

















