Developing a UHPLC Method for UV-based Detection and Quantification of Primary Aromatic Amines in Low Concentrations
The Application Notebook
Agilent Application Note
This application demonstrates that stringent sensitivity requirements for the detection of potentially harmful primary aromatic amines can be fulfilled when using an Agilent 1290 Infinity LC system equipped with the 1290 large volume injection kit.
Primary aromatic amines (PAAs) can originate from printing azodyes and azo-pigments. PAAs are potentially harmful and suspected to cause cancer; they have to be detected and determined.
Regulation (EC) 10/2011 sets a limit of 10 ppb for the sum of the released PAAs from plastic food contactmaterials. In the field of paper and board for food contact a similar limit is mentioned within BfR recommendation XXXVI. Moreover, the upcoming German regulation for printing inks used for food contact materials (Druckfarben V) negates the already introduced limit of 10 ppb for the sum of the released PAAs to all materials. Recently, problems have been reported within industry and enforcement authorities regarding the release of carcinogenic PAAs from heavily printed paper bags and napkins.
Since the legal limits apply to the sum of all PAAs, these compounds have to be detected down to a level of at least 1 ppb (1 ng/mL) for an individual PAA.
Experimental
To meet these requirements, two modifications were made to the standard configuration of the Agilent 1290 Infinity LC System. A 60-mm Max-Light high sensitivity flow cell was used with the 1290 Infinity Diode Array Detector. A 40-µL loop including the 1290 large volume injection kit to inject volumes up to 120 µL was used with the 1290 Infinity Autosampler.
The method that was finally applied was developed by using a mixture of six primary aromatic amines at 100 ng/mL: 1) aniline, 2) o-anisidine, 3) o-toluidine, 4) 2-methyl-5-nitroanilin, 5) 2, 4-dimethylanilin and 6) 2, 4-dichloranilin (Figure 1).
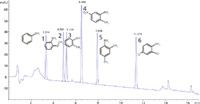
Figure 1: Primary aromatic amines at 100 ng/mL: 1) Aniline: 3.314 min, 2) o-Anisidine: 4.882 min, 3) o-Toluidine: 5.159 min, 4) 2-Methyl-5-nitroanilin: 6.488 min, 5) 2,4-dimethylanilin: 7.908 min and 6) 2,4-dichloranilin: 11.373 min.
The method starts with an enrichment step for the first minute followed by a steep increase to the starting conditions at 20% methanol. The gradient separation was done in the following 13 min up to 70% methanol on an Agilent ZORBAX Eclipse Plus C18, RRHT, 3.0 × 100 mm, 1.8 µm column.
Results
With the final method, a calibration was done for all PAAs from 100 ng/mL down to a level of 2 ng/mL (2 ppb). All linearity correlation coefficients were above 0.99983. The concentration level at 2 ng/mL was defined as the limit of quantification (LOQ signaltonoise ratio around 10). The limit of detection (LOD) was 0.5 ng/mL (signaltonoise ratio around 3).
A statistical evaluation was done on the 10 ng/mL level. The relative standard deviation (RSD) of the retention times was between 0.039% and 0.057%, the area RSDs were between 0.5% and 2.7%. Relative standard deviation of the compound response factors were between 0.004 and 0.017%.
To test the method, a blank matrix sample produced from a heavily coloured napkin was spiked with the six PAAs at various concentrations. All six PAAs could be well separated from the matrix compounds and identified and quantified with the developed method and calibration.
Conclusion
This application shows that the Agilent 1290 Infinity LC system can detect low amounts of primary aromatic amines by using the 60mm Max-Light high sensitivity cell in the diode array detector, and the large volume injection kit in the autosampler.
Agilent Technologies Inc.
5301 Stevens Creek Blvd., Santa Clara, California 95051, USA
tel. (800) 227-9770 (Directory), fax (866) 497-1134
Website: www.agilent.com

Silvia Radenkovic on Her Research and Passion for Scientific Collaboration
April 3rd 2025Radenkovic is a PhD candidate at KU Leuven and a member of FeMS. Her research focuses on inborn metabolic disorders (IMD), like congenital disorders of glycosylation (CDG), omics techniques such as tracer metabolomics, and different disease models.
Evaluating Natural Preservatives for Meat Products with Gas and Liquid Chromatography
April 1st 2025A study in Food Science & Nutrition evaluated the antioxidant and preservative effects of Epilobium angustifolium extract on beef burgers, finding that the extract influenced physicochemical properties, color stability, and lipid oxidation, with higher concentrations showing a prooxidant effect.


















