Enhanced Capillary Electrophoresis Performance using Dynamic Surface Coatings
LCGC Europe
The use of dynamic coating systems/approaches in CE can result in improved routine performance and/or selectivity.
A look at the use dynamic coatings to improve performance in capillary electrophoresis.
What are Dynamic Coating Approaches Used?
Improving repeatability/robustness: The migration time of a solute in capillary electrophoresis (CE) is dependent upon both the charge/size of solute ion and the level of electro-osmotic flow (EOF) generated in the capillary. The EOF is generated as a result of the charge on the capillary wall. In uncoated capillaries the negative charge is generated through dissociation of silanols on the capillary wall. Differences in the number of residual silanol contents between portions of capillaries from the same supplier and/or between capillaries from different suppliers leads to variable migration times and reduced method robustness. Use of a dynamically-coated system allows the amount of charge on the capillary wall to be closely controlled and consistent EOF levels and migration times across capillaries.
The level of EOF is also highly pH dependent as the silanols are acidic with a pKa value in the pH 4–5 region. The capillary wall therefore has a low charge at low pH (and a small/zero EOF). The EOF and charge are high at high pH values. Small day-to-day variations in pH in the mid-pH range cause large differences in the migration times obtained.
To reduce peak tailing: Solute adsorption is a significant problem for basic compounds as they are positively charged and are electrostatically attracted to the negatively charged capillary walls. This irreproducible adsorption can lead to peak broadening due to tailing, increased peak area variability and lower precision. Internal capillary surfaces can be chemically modified to give a permanent neutral, positive or negative charge which may assist in the elimination of peak tailing and/or alter the speed/direction of EOF. However, these internally coated capillaries have increased costs compared to uncoated capillaries and attention must be paid to the repeatability, stability and durability of the coatings. Dynamicallycoated capillaries can reduce adsorption and tailing issues and do not suffer from stability or durability issues.
Manipulating EOF direction/speed and selectivity: Surface active additives such as cationic surfactants can alter the speed/direction of the EOF. This can beneficially affect selectivity and/or decrease analysis time. This is discussed later specifically for the analysis of inorganic anions.
What Dynamic Coating Approaches Are Used?
Dynamic coating systems: Coating systems have been developed which reproducibly dynamically coat the internal wall of the capillary. These are available commercially (1,2) or the components can be purchased independently (3) and used. Typically, a three-stage process is employed which initially involves flushing the capillary with NaOH solution to fully dissociate the surface silanols and generate a highly negatively-charged surface. An initiator solution, which contains a polyamine substance, is then flushed through the capillary. The multiply charged polyamine adsorbs onto the capillary wall which results in a complete and uniform coating and positive charge. The capillary is finally flushed (initiated) with a buffer solution at the required pH which contains a polyanion that gives (Figure 1) a uniform coating and a consistent negative charge to the capillary. The negative charge of the polyanion layer is unaffected by pH variation as the polyanion contains sulphonic acid groups that remain ionized over the entire pH range.
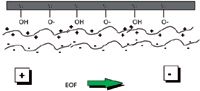
Figure 1
The buffering agents generally used in these systems (1,2) are zwitterionic amino acids such as Tris, arginine and taurine. The buffers have a low ionic strength and a good buffering capacity. The low ionic strength is of benefit as this reduces the operating current compared to standard inorganic buffers used in CE such as phosphate. The lower currents generated permit use of either wider bore capillaries for improved sensitivity or higher applied voltages.
Migration times are also reduced for the basic compounds at low pH values as the electrophoretic migration of the solutes is supplemented by an appreciable EOF. This combination of EOF and mobility can reduce migration times by up to 50%.
A very systematic evaluation of the factors affecting the dynamic coating stability has been conducted (4) on non-commercial coating approaches.
Surface active agents: These include polybrene (hexadimethrine bromide) (5) and TTAB (tetradecyl trimethyl ammonium bromide) (6). Figure 2 shows the process and the effect obtained. The cationic surfactants ion-pair onto the negatively-charged capillary wall forming a mono-layer. The long-chain tails of the surfactants then interact to form a bi-layer. The bi-layer results in the capillary wall having an effective positive charge which causes the EOF flow to change direction compared to an uncoated capillary. The positively charged capillary wall repels cationic solutes which (5) eliminates peak tailing. Relatively low concentrations of cationic surfactants are employed (6) (for example, 0.5 mM TTAB) as micelles are formed at higher concentrations which dramatically alter the separation selectivity.

Figure 2
What Levels Of Improvement Can Be Obtained With These Coating Approaches?
Repeatability improvements: An experimental piece of glass tubing was obtained (1) which contained nineteen 25-µm channels. The benefits of employing this capillary format would be that improved injection loadings and sensitivity could be obtained compared to a standard wider bore capillary. The current (and heat) generated in each 25 µm channel would be low as heat should be effectively dissipated.
Figure 3(a) shows the capillary used to analyse a solution of the bronchodilator salbutamol with a standard phosphate pH 2.5 buffer. This produced a series of split peaks due to different levels of EOF being generated in each channel. Figure 3(b) shows (1) the analysis of the same sample solution on the multi-bore capillary using a pH 2.5 polyanionic buffer after dynamically coating the surface. A single, higher intensity peak was generated which shows that the EOF is uniform across each of the channels. The peak time is reduced in Figure 3(b) due to the EOF present. This example particularly exemplifies the dramatic improvement in repeatability using a dynamic coating system.

Figure 3
Across-Capillary Reproducibility: Control of EOF between capillaries is problematic as it relies on the silanol density which changes between capillaries. Extensive rinsing of the capillaries with NaOH to activate the silanols is recommended to improve reproducibility but variability still occurs which detracts from use in a routine quality control (QC) environment. Figure 4 shows separation (1) of 5 basic compounds on 3 freshly prepared capillaries. All capillaries were rinsed for 10 min with 0.1 M NaOH followed by 1 min with the polyamine solution and then 2 min with the pH 2.5 polyanionic buffer. All capillaries gave an operating current of 16 or 17 µA. The separations shown in Figure 4 were the first injection on each new capillary. The reproducibility of migration times for the 3 separate capillaries is considerably better than the repeatability for 3 normal, untreated CE capillaries using a conventional buffer – this is especially important in routine QC operations.

Figure 4
Injection precision: Table 1 shows data (1) from studies measuring precision. Injection sequences were performed using a test-mixture containing 3 basic drugs and aniline, which is used as an internal standard. These measurements were conducted on different days and on 3 separate capillaries. The improved peak shape, reduced tailing and improved peak time repeatability made accurate and more reproducible integration possible. The inherent injection volume variability in CE is shown in the 2–3% relative standard deviation (RSD) values obtained for peak areas (Table 1). This injection volume variability is successfully counteracted by the use of the internal standard approach, that is, the peak area ratio (PAR) values were calculated.
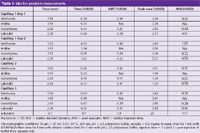
Table 1: Injection precision measurements.
What Applications Have Dynamically Coated Capillaries Been Used For?
Figures 3 and 4 show that the dynamic coating systems can be used (1) for routinely analysing basic drugs. Commercial coating systems covering a range of pH values are available and allow for example separation of acidic species.
The low pH dynamic coating systems have been used (2) for fast screening of tobacco-specific N-nitrosamines (TSNA's), which are carcinogens. The reported method was faster, simpler and lower cost than the previously used methodology. The method was used to measure TSNA levels in serum samples.
Dynamic coating buffers have been successfully applied to protein analysis (4) with intra-day RSD's of less than 1% being obtained with very good selectivity. Similar performance levels have been reported (3) for a range of peptides analysed using dynamicallycoated capillaries.
Polybrenecoated capillaries have been used (5) to monitor nucleotide pyrophosphates/phospodiesterases reactions with various inhibitors. Sample throughput was high. For example, ATP, UMP and AMP were monitored with an analysis time of 5 min.
The most common and frequent use of dynamic coated capillaries is in the determination of inorganic anions, such as chloride and sulphate, and simple organic acid ions such as citrate and maleate. TTAB for example (6) is added to the buffer to generate a negative EOF direction which matches the migration of the small negativelycharged solutes. A negative voltage is then used in separation mode. These methods are very robust and have found (6) routine use in a number of industries, including pharmaceuticals, agrochemicals and application in food analysis and forensics.
Conclusions
The use of dynamic surface coating approaches can significantly improve the routine performance of capillary electrophoresis. Commercial and homemade dynamic coating systems are easy to use and significantly improve the repeatability of migration times between capillaries. The coating systems are available to cover a range of applications and are widely used in routine QC operations in many industries.
Dynamic coatings are also useful for altering the selectivity and optimizing robust operating performance. This is particularly illustrated in the analysis of inorganic anions and simple acids where the methods developed are routinely applied across a wide number of industries and applications.
Kevin Altria is an associate director in the pharmaceutical development department of GlaxoSmithKline. He is editor of "CE Currents" and a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should go to "CE Currents", LCGC Europe, Advanstar Communications, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or email the LCGC Europe editor, Alasdair Matheson, at amatheson@advanstar.com
References
(1) K.D. Altria, J. Pharm Biomed Analysis, 31, 447–453 (2003).
(2) J. Liao, Y. Pan, C. Li, D. Wen and H. Liu, Chromatographia, 74, 415–419 (2011).
(3) R. Nehme, C. Perrin, H. Cottet, M.D. Blanchin, H. Fabre, Electrophoresis, 29, 3013–3023 (2008).
(4) R. Nehme, C. Perrin, H. Cottet, M.D. Blanchin, H. Fabre, Electrophoresis, 30, 1888–1898 (2009).
(5) J. Iqbal, S.A. Levesque, J. Sevigny, C. Muller, Electrophoresis, 29, 3685 (2008).
(6) K.D. Altria, LCGC Europe, 24(1), 32–36 (2011).
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.












