Empower 2 Method Validation Manager Software: A Tool for Rapid Method Validation
Special Issues
Method validation is a demanding activity. It requires a large investment in personnel, materials, instruments, supervision, and, most of all, time.
APPLICATION BENEFITS
This application note summarizes a more comprehensive piece on the efficiencies and productivities to be gained by use of Empower™ 2 Method Validation Manager Software.
WATERS SOLUTIONS
Empower 2 Method Validation Manager
ACQUITY UPLC®
KEY WORDS
Method development, method validation, validation protocols, sample lists, compliance, data security
INTRODUCTION
Method validation is a demanding activity. It requires a large investment in personnel, materials, instruments, supervision, and, most of all, time. Some of the more time-consuming aspects of validation involve the creation of validation protocols and sample lists, tracking of the workflow from protocol to final reporting, the performance of calculations, and the intense need to organize and manage raw and processed data. The potential for errors in the many steps of the validation process is large and the time delays when errors occur can be costly.
Waters Empower 2 Method Validation Manager (MVM) Software, used in partnership with the ACQUITY UPLC System, can dramatically address these time-consuming elements of analytical method validation. MVM is designed to streamline the setup, execution, calculation, and reporting of a method validation. It provides easy data tracking and complete organization of validation data and results monitored by the built-in oversight of automated error checking.
MVM is business-critical software that can significantly reduce the time and costs required to perform chromatographic method validation. Because MVM allows the entire chromatographic method validation process to be efficiently performed within Empower 2, fewer software applications need be deployed, validated, and maintained. Software training and support is also minimized. When less software is required, the software that is business-essential can be deployed more quickly and efficiently.
In addition, Method Validation Manager allows you to be fully compliant with governmental regulations by providing data security, a full set of user privileges, audit trails, and automatic data documentation; providing you with the necessary information and complete data traceability required for final reports and to pass audits and data reviews.
To illustrate the straightforward operation and comprehensive functionality of MVM, the major steps involved in the workflow for a basic assay validation of the drug product acetaminophen are summarized in this work.
DISCUSSION
Method system suitability criteria
Here we illustrate the utility of MVM software in the validation of a method for the analysis of acetaminophen. The method system suitability criteria for the analysis of acetaminophen are listed in Table 1. The full method is described in the extended version of this Waters Application Note (2007; 720002401en), available on the Waters website.

Table 1. Method suitability criteria.
Acetaminophen analysis with the ACQUITY UPLC System and an ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 50 mm Column, is shown in Figure 1.
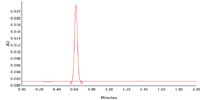
Figure 1. Analysis of acetaminophen.
Validation protocol and execution
The elements of the written validation protocol for this method were easily transferred into the validation protocol method template of MVM (Figure 2).
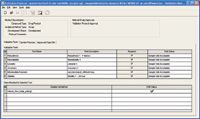
Figure 2. The written protocol can be easily transferred to Empower MVM.
The following validation tests were performed in this study:
- Robustness (for three factors)
- Repeatability
- Intermediate precision (different analyst)
- Linearity
- Accuracy
- Solution stability (24 hours)
(See the extended version of this piece for all data examples.)
Individual tests and their associated acceptance criteria were configured.
For the accuracy test configuration, three specified concentration levels with three individual preparations were injected one time. Levels were entered in the levels table. MVM then ensures that samples submitted for accuracy analysis match the defined parameters. For the acceptance criteria for the accuracy test, percent recovery was the test result of interest. The acceptance range for the test is indicated as 95 to 105%.
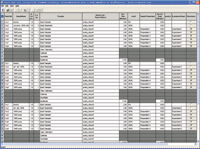
Figure 3. Sample set method.
Complete sample set methods were constructed and then saved as templates within the validation protocol method, as shown in Figure 3.
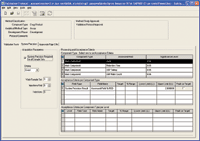
Figure 4. System precision parameters. In this protocol, the % RSD of peak area must be no more than 2%.
Since the validation protocol called for method suitability parameters to be met by each analysis, system precision requirements were also configured, as shown in Figure 4.
During the process of test configuration and sample set method construction, errors were automatically caught by MVM, illustrated by a red X in the validation protocol window. Using the update status button and responding to error messages from the message center effectively guides all troubleshooting activity. Thus MVM ensures that all sample set methods are consistent with their respective test configurations.
The validation protocol method was saved within a validation template project. Next, a validation working project was started and a new study was initiated based on the validation protocol method template.
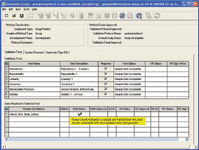
Figure 5. The green check mark in this validation manager window indicates that the sample set method is consistent with the user-configured test criteria.
The validation manager window lists the test configurations and acceptance criteria for the validation study. Additional functionality includes indicators that show test status and required approval (Figure 5).
Confirmation of MVM-driven study
Since complete sample set methods are contained in the validation protocol method, the study can now be executed. Standards and samples were prepared then analyzed on the ACQUITY UPLC System as the previously established sample set methods.
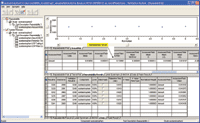
Figure 6. Repeatability, or intra-assay precision, test result in the validation result review window.
The analytical method for acetaminophen was evaluated for accuracy, repeatability, intermediate precision, linearity, robustness and stability. All parameters met their predefined acceptance criteria. Examples of the accuracy and linearity screens from MVM are shown in Figures 6 through 9.
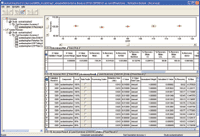
Figure 7. Accuracy validation result review window.
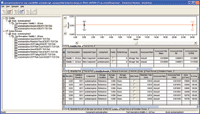
Figure 9. The consolidated results of two separate sample sets are presented in the stability validation test result review window.
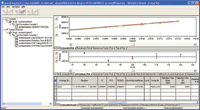
Figure 8. Linearity result shown in the validation result review window.
Validation summary
The status and final results for each of the validation tests was clearly displayed in the validation manager window. The green checks indicated tests with acceptable validation results, while the yellow triangle flagged robustness test results that fell outside the acceptance range (Figure 10).
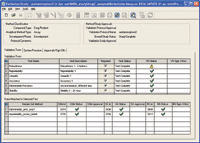
Figure 10. The validation manager window shows that validation is complete.
The method for the assay of acetaminophen was analyzed for robustness, repeatability, intermediate precision, accuracy, linearity, and solution stability. This assay was found to be linear, accurate, repeatable, and to be accurately and precisely performed by more than one analyst.
Additionally, samples prepared following the method procedure were documented as stable for 24 hours. From the robustness testing, altering the column temperature and flow rate was found to significantly affect the accuracy and precision of the method. The method will be revised to clearly state the need to control these two factors.
CONCLUSION
Empower 2 Method Validation Manager Software effectively streamlines the validation process and integrates smoothly into the validation workflow of the compliant laboratory.
Some of the benefits from the use of MVM are:
- Regulatory compliance: Empower 2 MVM Software easily meets all of the regulatory needs of the compliant laboratory.
- Straight-forward validation troubleshooting: The update tool/message center provides an application-directed, time-efficient troubleshooting process, reducing the time required to get the validation back on track.
- Data traceability: Out of specification results are clearly indicated and subsequent investigations are facilitated by the self-contained, completely traceable data management capability of the MVM.
- Reduction of supervisory review: The onus of supervisory review is reduced using MVM, enabling rapid progression in the validation workflow. Potentially error-prone steps such as processing, calculation, and overall data management are all eliminated with the automatic, self-contained design of MVM. The need for any additional third party software packages is also eliminated.
- Validation consistency: The ability to create project and sample set method templates ensures consistency of validation protocols with the guidance documents of the laboratory. This reduces errors in the execution of the protocols and increases confidence in the data acquired and the results obtained.
MVM not only effectively organizes and manages the performance of a method validation; it also delivers inarguable confidence in its results. Coupling Empower 2 Method Validation Manager Software to the ACQUITY UPLC System provides an unparalleled solution to the validation needs of a laboratory.
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990

Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
The Effect of Time and Tide On PFAS Concentrations in Estuaries
April 8th 2025Oliver Jones and Navneet Singh from RMIT University, Melbourne, Australia discuss a recent study they conducted to investigate the relationship between tidal cycles and PFAS concentrations in estuarine systems, and offer practical advice on the sample preparation and LC–MS/MS techniques they used to achieve the best results.









