The Effect of Draw-Out Lens Diameter on Sensitivity of GC–MS Analysis
The Application Notebook
Gas chromatography–mass spectrometry (GC–MS) allows isolation and identification of individual analytes within a complex mixture. Helium has traditionally been the first-choice carrier gas, owing to its inertness, performance, and relatively cheap price. Since 2001, however, helium has become increasingly expensive with a reported global increase in price of 500% between 2001 and 2016 (1). In 2012–2013, the global helium shortage increased the number of GC users switching to alternative carrier gases and improved the availability of information on their use.
Ed Connor1 and Carlos Fidelis2, 1Peak Scientific Instruments, 2Department of Chemistry, UNICAMP Sao Paolo, Brazil
Gas chromatography–mass spectrometry (GC–MS) allows isolation and identification of individual analytes within a complex mixture. Helium has traditionally been the first-choice carrier gas, owing to its inertness, performance, and relatively cheap price. Since 2001, however, helium has become increasingly expensive with a reported global increase in price of 500% between 2001 and 2016 (1). In 2012–2013, the global helium shortage increased the number of GC users switching to alternative carrier gases and improved the availability of information on their use.
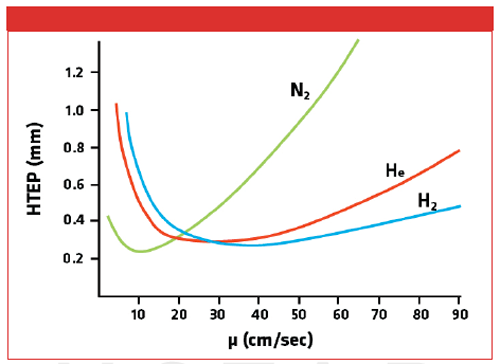
Hydrogen is half as viscous as helium at the same temperature and pressure, while the diffusion of a sample within the two gases is similar, meaning that hydrogen travels through the GC column more quickly and offers faster analysis than helium. The van Deemter curve (Figure 1) shows the relative efficiencies of hydrogen, helium, and nitrogen at different flow rates and shows how hydrogen has superior column efficiency at higher flow rates. Using method translation software (2,3), it is possible to model the effect of converting a method from helium to hydrogen in silico to see what time savings can be made and what changes to the method are required.
Figure 1: van Deemter curve showing the relative efficiencies of hydrogen, helium, and nitrogen at different flow rates.
To overcome concerns about sensitivity reduction and stabilization times associated with hydrogen carrier gas, hardware changes such as increasing the draw-out lens orifice diameter and baking out the ion source can be conducted. Following analysis of an essential oil mixture run using helium carrier gas with a standard ion volume and hydrogen carrier gas using a larger diameter draw-out lens across a range of flow rates, stabilization time, peak resolution, and signal-to-noise ratio were assessed.
Materials and Methods
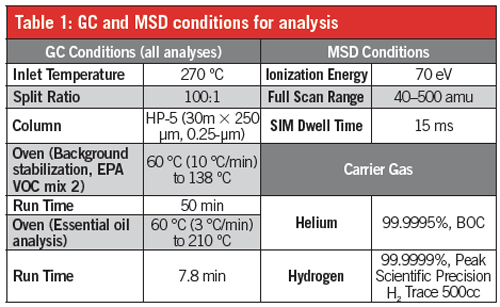
Gas Chromatography–Mass Spectrometry (GC–MS) Analysis: All GC–MS analyses were performed using an Agilent Technologies 7890B GC with 5975 mass selective detector. Table 1 shows GC and MSD conditions for essential oils provided by Professor Lauro Barata from UFOPA (Universidade Estadual do Oeste do Pará)VOC mix was purchased from Supelco (EPA VOC Mix 2).
Analyses of samples run using helium carrier gas were acquired using an inert 3 mm draw-out plate (G2589-20100). All samples run using hydrogen carrier gas were acquired using an inert 6 mm draw-out plate (G2589-20045).
Background stabilization was assessed by running a volatile organic mixture for 7 days following change of carrier gas. The ion source was baked-out using a slight modification of recommendations (p35–37) by Agilent Technologies (4), with the source temperature set to 300 °C and filament switched on for a period of 3 h.
Results
Effect of Carrier Gas on Signal to Noise: Signal to noise (S/N) and resolution (Rs) were calculated using 1,3,5-trichloro benzene, the last eluting peak of an essential oil mixture (Table 2). Samples run with helium carrier gas in full scan mode showed an inverse relationship between flow rate and S/N, with S/N dropping from 1988.3 at the optimal 1.0 mL/min flow rate to 864.9 at 2.0 mL/min (Table 2). When running samples using hydrogen carrier gas, the opposite relationship between flow rate and S/N was found, with S/N increasing from 106.0 to 209.6 as column flow increased (Table 2).
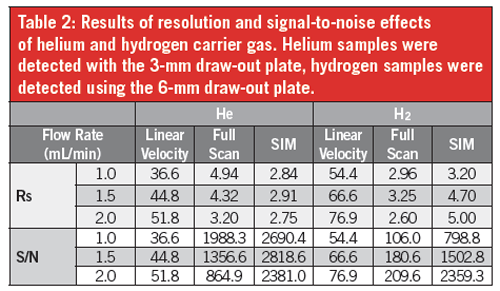
In SIM mode, there was little variation in S/N regardless of flow rate when using helium carrier gas. However, with hydrogen carrier gas, S/N improved with increasing carrier flow rate, with the S/N increasing from 798.8 at 1.0 mL/min to 2359.3 at 2.0 mL/min, meaning that S/N with hydrogen at higher flow rates was almost the same with helium (Table 2).
Effect of Carrier Gas on Resolution: In full scan mode, helium carrier gas followed a similar pattern to S/N results, with Rs decreasing as flow rate increased beyond the optimal velocity. When running samples using hydrogen, there was no clear relationship between Rs and flow rate, with the best resolution seen at 1.5 mL/min. When comparing the optimal flow rates of each gas (1.0–He and 2.0–H2), peak resolution with helium carrier gas was almost double (1.9×) that of hydrogen (Table 2).
In SIM mode, Rs when using helium decreased relative to full scan Rs and was lower than Rs seen with hydrogen carrier gas (Table 2). Hydrogen Rs was vastly improved in SIM mode compared with scan mode (1.9×) and at optimal flow rates, hydrogen gave improved Rs (1.76×) compared to helium.
Background Stabilization: Results showed that background was stable after 3 days, with repeated injections of the EPA VOC mixture being tested for 7 days (Figure 2).
Table 2: Results of resolution and signal-to-noise effects of helium and hydrogen carrier gas. Helium samples were detected with the 3-mm draw-out plate, hydrogen samples were detected using the 6-mm draw-out plate.
Discussion
A number of applications use hydrogen carrier gas as a viable alternative to helium. The results of GC–MS when comparing Rs and S/N appear to correspond directly to the carrier gas flow rate relative to the optimal carrier gas velocity of both gases. At an optimal column flow of helium, the best performance for both Rs and S/N were observed in full scan mode. SIM detection appeared to overcome some of the problems of reduced carrier gas efficiency of helium at higher velocities, with little difference found in either Rs or S/N across the range of flow rates tested.
Similarly to helium, running samples with hydrogen carrier gas at a suboptimal flow rate affected Rs and S/N significantly in both scan mode and SIM mode. Interestingly, hydrogen carrier gas showed better peak Rs in SIM mode than helium across all three flow rates. It appears that running in SIM mode largely eliminates background noise that causes interference in full scan mode when using hydrogen. When following the recommendations for preparation of the system when switching to hydrogen, background signal will take at least 3 days to stabilize.
This study clearly demonstrates that hydrogen can be used for routine analysis of known compounds. When using full scan mode, analysts need to be aware that they are likely to see a two- to fivefold reduction in sensitivity (4). When using hydrogen carrier gas for GC–MS, it is essential to initially focus on mitigation of factors causing reduced sensitivity.
References
- https://www.theguardian.com/science/2016/jun/28/huge-helium-gas-tanzania-east-africa-averts-medical-shortage
- http://www.restek.com/ezgc-mtfc
- http://www.agilent.com/en-us/support/gas-chromatography/gcmethodtranslation
- http://bit.ly/2rqxQQu

Peak Scientific
Fountain Crescent, Inchinnan Business Park, Inchinnan, PA4 9RE, Scotland, UK
E-mail:info@peakscientific.comWebsite:www.peakscientific.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.