Determination of Pesticides in Coffee with QuEChERS Extraction and Silica Gel SPE Cleanup
The Application Notebook
Coffee is one of the most widely consumed beverages in the world, partly because of the stimulating effect of its caffeine content. Like most crops, the application of pesticides in coffee cultivation is a common practice to increase production yields. This application note details an optimized method for the extraction and cleanup of pesticide residues from coffee using a QuEChERS extraction procedure followed by a silica gel solid-phase extraction (SPE) cleanup.
Xiaoyan Wang, UCT, LLC
Coffee is one of the most widely consumed beverages in the world, partly because of the stimulating effect of its caffeine content. Like most crops, the application of pesticides in coffee cultivation is a common practice to increase production yields. This application note details an optimized method for the extraction and cleanup of pesticide residues from coffee using a QuEChERS extraction procedure followed by a silica gel solid-phase extraction (SPE) cleanup.
Procedure
1. Sample Extraction
a) Add 10 mL brewed coffee (pH adjusted to about 8 with 1 N NaOH) and 10 mL acetonitrile (MeCN) to a 50-mL centrifuge tube.
b) Add the QuEChERS extraction salts from the Mylar pouch (ECMSSC50CT-MP) to the 50-mL tube, and shake vigorously for 1 min manually or using a
Spex 2010 Geno-Grinder at 1000 strokes/min.
c) Centrifuge at ≥3000 rcf for 5 min.
d) Transfer 5 mL supernatant to a clean test tube, add 1.5 mL toluene, and evaporate to about 1 mL.
2. Sample Cleanup of Extract
a) Add about ½ inch of anhydrous sodium sulfate to a silica gel SPE cartridge (CUSIL156), and attach the SPE cartridge to a glass block or positive
pressure manifold.
b) Wash the SPE cartridge with 6 mL dichloromethane, soak for 1 min, drain to waste, and dry the SPE cartridge for 1 min under full vacuum or pressure.
c) Condition the SPE cartridge with 2 × 6 mL hexane by gravity.
d) Insert a glass collection container into the manifold, load the 1 mL concentrated sample onto the SPE cartridge, rinse the test tube with 6 mL of 15%
acetone in n-hexane, apply the rinsate to the SPE cartridge, and collect.
e) Continue to elute with 3 × 6 mL of 15% acetone in n-hexane by gravity.
f) Add 1.5 mL ethyl acetate to the eluate container and evaporate to 1 mL.
g) Add internal standard, vortex for 30 s, and inject 1 μL into the GC–MS system for analysis.
Instrumental
GC–MS/MS: Agilent 6890N GC coupled to a 5975C MSD
Column: 30 m × 0.25 mm, 0.25-µm Restek Rxi®-5Sil MS
Carrier Gas: Helium (1.2 mL/min)
GC Inlet Temperature: 250 °C
Injection Volume: 1 μL (splitless)
Temperature Gradient: 60 °C for 1 min, 10 °C/min to 310 °C, hold for 2 min; 28 min total
Ion Source Temperature: 250 °C
Ionization Mode: EI (70 eV)
Acquisition Mode: SIM
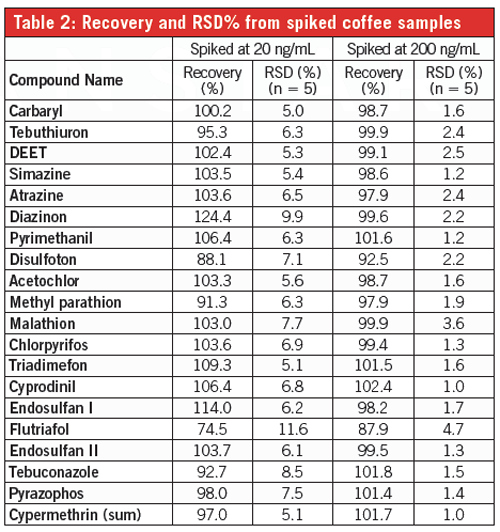
Results

UCT, LLC
2731 Bartram Road, Bristol, Pennsylvania 19007, USA
Tel: (800) 385 3153
E-mail:methods@unitedchem.comWebsite:www.unitedchem.com

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)