Drug-Antibody Ratio of an Antibody–Drug Conjugate by SEC-MALS
The Application Notebook
Drug-antibody ratio (DAR) is a critical attribute of antibody–drug conjugates (ADCs). DAR may often be determined quickly and effectively by combining SEC with three online detectors: MALS, UV, and dRI.
Drug-antibody ratio (DAR) is a critical attribute of antibody–drug conjugates (ADCs). DAR may often be determined quickly and effectively by combining SEC with three online detectors: MALS, UV, and dRI.
There has been a significant resurgence in the development of antibody–drug conjugates (ADC) as target-directed therapeutic agents for cancer treatment. Among the factors critical to effective ADC design is the drug-antibody ratio (DAR), which describes the degree of drug appended to the antibody and directly impacts both potency and potential toxicity of the therapeutic. In addition to therapeutic and physiological effects, DAR can also affect drug product properties such as stability and aggregation. Determination of DAR is, therefore, of critical importance in the development of novel ADC therapeutics.
DAR is typically assessed by mass spectrometry (MALDI-TOF or ESI-MS) or UV spectroscopy, both of which are subject to certain limitations: 1) calculations based on UV absorption are often complicated by similarities in extinction coefficients of the antibody and small molecule; 2) mass spectrometry, though a powerful tool for molecular weight determination, depends on uniform ionization and recovery between compounds-which is not always the case for ADCs. We present here a method for DAR determination based on SEC-MALS in conjunction with UV absorption and differential refractive index (dRI) detection, which overcomes these limitations for many ADCs.
Experimental Conditions
Two model ADCs based on the same monoclonal antibody and drugâlinker system, but different conjugation processes, were analyzed. In this example the antibody has been alkylated with a compound having a nominal molar mass of 1250 g/mol.
Each ADC was passed through size-exclusion chromatography (SEC) followed by the HPLC’s UV detector, a DAWN multi-angle light-scattering (MALS) detector (Wyatt Technology) and an Optilab differential refractive index (dRI) detector (Wyatt Technology). Protein molar mass (Mprot), molar mass of the drug and linker (Mdrug), and total molar mass (Mcomplex) of the ADC were determined at each elution volume by analysis of signals from the three detectors.
Data collection and analysis were performed in the ASTRA software (Wyatt Technology) using ASTRA’s Protein Conjugate Analysis method. Conjugate analysis is automated within ASTRA using the differential refractive index increments (dn/dc) and UV extinction coefficients, which are determined empirically for each species or mined from the literature.
Results
Figure 1 shows UV traces for the two model ADCs along with the calculated values of Mcomplex, Mdrug, and Mprot of each. As expected, the molar masses of the antibody components are quite similar; but Mdrug differs considerably. Despite the drug constituting just a small fraction of overall molar mass, this highly sensitive system shows robust Mdrug values which are well-distinguished from each other and vary little over the peak. The inset presents just Mcomplex, clearly showing the sensitivity of the system which differentiates the two molecules quite well.
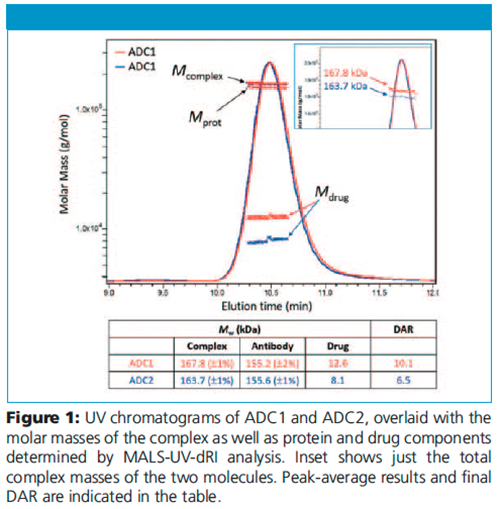
Final DAR is calculated from the ratio of Mdrug to the molar mass of a single drug + linker compound, shown in the table. The results are consistent with values obtained by orthogonal techniques. Note that the molar mass traces for the conjugated moiety represent the total amount of attached pendant groups; the horizontal trends indicate that modification is nearly uniform throughout the population eluting in that peak.
This analysis was successful thanks to the relatively large total appended mass, about 5% (ADC2) to 8% (ADC1) of the total molar mass of the complex. In general, for accurate SEC-MALS-UV-dRI protein conjugate analysis, the modifier mass should be at least 3% of the total. Hence a lower degree of conjugation might not have been amenable to analysis by this method.
Conclusions
SEC-MALS-UV-dRI provides an easy, accurate means to determine DAR of ADCs in the course of product development and quality control. It is suitable for relatively high DAR values where the total drugâlinker mass is at least 3% of the overall complex mass.

Wyatt Technology
6330 Hollister Avenue, Santa Barbara, California 93117, USA
Tel.: +1 (805) 681 9009
Website: www.wyatt.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.





