Disinfection Byproducts in Natural Waters
The Column
The occurrence of disinfection byproducts in natural waters poses a health risk for humans as well as aquatic organisms. This article presents a method, which was recently developed at the University of Arizona, in Tucson, Arizona, USA, for the fast and simultaneous determination of 15 regulated and unregulated disinfection byproducts.
Photo Credit: Anon samersa/Shutterstock.com

The occurrence of disinfection byproducts in natural waters poses a health risk for humans as well as aquatic organisms. This article presents a method, which was recently developed at the University of Arizona, in Tucson, Arizona, USA, for the fast and simultaneous determination of 15 regulated and unregulated disinfection byproducts.
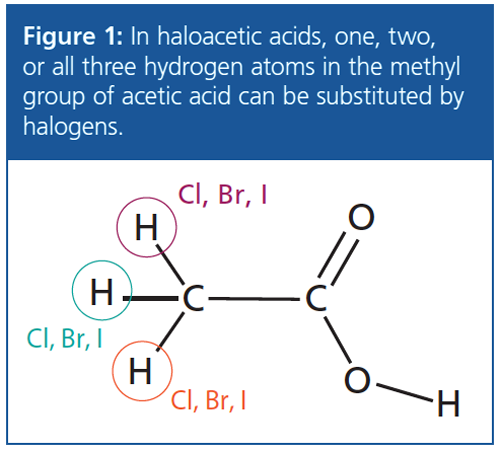
Disinfectants used in water treatment, including chlorine and bromine, can react with natural organic matter and anthropogenic contaminants in the water to form disinfection byproducts (DBPs). Haloacetic acids (HAAs), including dichloroacetic acid, are common DBPs which have been classified as possibly carcinogenic to humans by the US Environmental Protection Agency (EPA) as well as the International Agency for Research on Cancer (IARC) (Figure 1).
DBPs have been a subject of concern because of their adverse biological effects, not only on humans, but also on aquatic organisms (1). Coca-Cola has had to recall 500,000 bottles of its Dasani water in the UK because it was contaminated with bromate, another DBP (2). Removing contaminants in drinking water while avoiding the formation of disinfection byproducts is a balancing act where efficacy must be tested by monitoring DBP concentrations in water treated with disinfectants. A new ion chromatography–tandem mass spectrometry (IC–MS/MS) method enables the fast and simultaneous analysis of various DBPs in samples of disinfected water.
Health Concerns Associated With DBPs
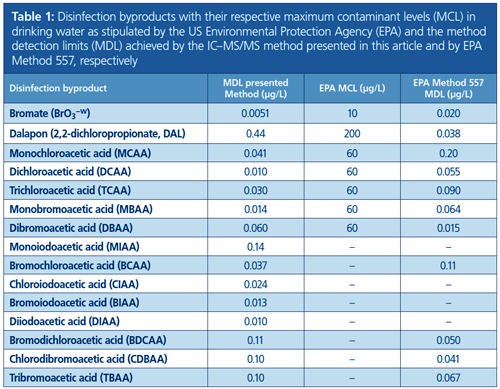
The EPA stipulates limit values for several DBPs. These include bromate (BrO3−) and dalapon (2,2-dichloropropionate, DAL) as well as five HAAs, namely monochloroacetic acid (MCAA), dichloroacetic acid (DCAA), trichloroacetic acid (TCAA), monobromoacetic acid (MBAA), and dibromoacetic acid (DBAA), which are referred to as HAA5. In addition, four more HAAs have been commonly monitored in the past decade: tribromoacetic acid (TBAA), bromochloroacetic acid (BCAA), bromodichloroacetic acid (BDCAA), and chlorodibromoacetic acid (CDBAA). Together with the HAA5, these form the HAA9. EPA Method 557, which uses IC coupled to electrospray ionization tandem MS (IC–ESI–MS/MS), is dedicated to the determination of these nine HAAs as well as bromate and dalapon in natural waters.
Recently, the iodinated counterparts of the above-listed HAAs caused a public health concern because they appear to be even more toxic than the chlorinated and brominated HAAs (3,4). The substances in question are monoiodoacetic acid (MIAA), chloroiodoacetic acid (CIAA), bromoiodoacetic acid (BIAA), and diiodoacetic acid (DIAA). However, few quantitative methods have been described that enable the simultaneous analysis of these four iodinated HAAs. A new article recently published by Wu et al. in the Journal of Chromatography A (5) describes a method that uses IC–MS/MS to measure the concentrations of all substances targeted by EPA Method 557 as well as the four iodinated substances listed above simultaneously. Method detection limits for all analytes are at the sub-µg level and well below the respective maximum contaminant levels. Meanwhile, no sample preparation steps are necessary and, with 27 min measurement duration, the analysis takes just half as long as EPA Method 557.
Method
Instrumentation: The presented method, like EPA Method 557, relies on IC–ESI–MS/MS. The IC system consists of an ion chromatograph and a sample processor for automation (both from Metrohm). The system is also equipped with in-line ultrafiltration, which protects the analytical column from damage caused by particulate matter contained in the natural water samples. A triple quadrupole mass spectrometer (Agilent 6490 triple quadrupole mass spectrometer with Jet Stream dual electrospray source and iFunnel technology) is coupled to the ion chromatograph (Metrohm 850 Professional IC with 858 Professional Sample Processor and Metrohm Inline Ultrafiltration module).
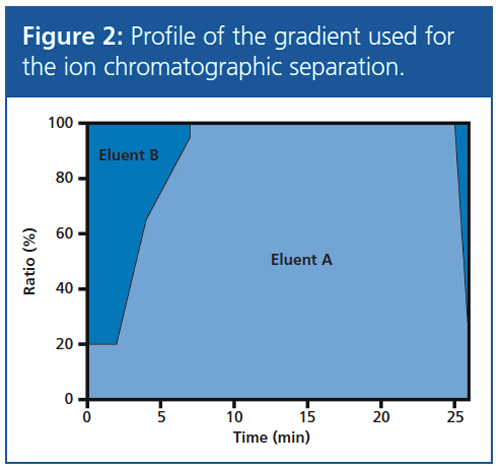
Ion Chromatographic Separation: The separation was performed on a 250 × 4.0 mm Metrosep A Supp 7 column (Metrohm) using gradient elution to obtain optimum separation of the weakly retained analytes–BrO3−, MCAA, MBAA, and MIAA–and to accelerate the elution of the strongly retained analytes. At the outset, only 20% (v/v) of eluent A is used, which consists of 50 mM KOH and 7 mM Na2CO3 in water:acetonitrile (85:15, v/v). The remaining 80% (v/v) of the eluent is made up of eluent B (ultrapure water). Elution was then accelerated by gradually increasing the proportion of eluent A. The profile of the gradient can be seen in Figure 2. The acetonitrile added to eluent A further quickens the elution by weakening hydrophobic interaction at the ion exchanger material.
Method Performance and Results
The method detection limits (MDLs) obtained with the new IC–MS/MS method are well below the the EPA limit values for all analytes and in many cases even below the MDLs of EPA Method 557. In Table 1, the MDLs of all target analytes are compared to the MDLs of EPA Method 557 and the limit values stipulated by the EPA. For all analytes, high intra- and inter-day precisions were achieved at 0.9–4.4% and 3.2–7.3% (relative standard deviation), respectively.
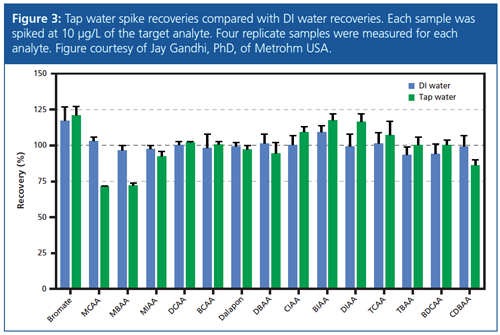
The recoveries, which were in the range of 77–125% for finished drinking water and 81–112% for surface water, are well within the accepted range of 70–130% stipulated by the EPA for the analysis of HAAs and dalapon (6). Figure 3 shows the tap water matrix spike recoveries of all target analytes in comparison with DI water spike recoveries. Each sample was spiked at 10 µg/L of the target analyte. Four replicates of each sample were measured.
Conclusion
IC–MS/MS can be used to determine many DBPs at once without requiring time-consuming sample preparation steps. It is currently the method of choice for the determination of HAAs, as well as dalapon and bromate, according to EPA Method 557. The new method developed by Wu et al. makes DBP analysis even better: while simultaneously determining all target analytes of EPA Method 557 as well as four additional substances–iodinated HAAs that are suspected to be highly cytotoxic and genotoxic–it cuts analysis time in half.
References
[1] J.A. Pals, J.K. Ang, E.D. Wagner, et al., Environ. Sci. Technol.45, 5791–5797 (2011).
[2] Coke recalls controversial water. (19 March 2004). BBC News. Retrieved from http://news.bbc.co.uk
[3] M.J. Plewa, E.D. Wagner, S.D. Richardson, et al., Environ. Sci. Technol. 38, 4713–4722 (2004).
[4] S.D. Richardson, F. Fasano, J.J. Ellington, et al., Environ. Sci. Technol. 42, 8330–8338 (2008).
[5] S. Wu, T. Anumol, J. Gandhi, et al., J. Chromatogr. A 1487, 100–107 (2017).
[6] USEPA Method 552.2 “Determination of haloacetic acids and dalapon in drinking water by liquid–liquid extraction, derivatization and gas chromatography with electron capture detections, revision 1.0,” in Methods for the Determination of Organic Compounds in Drinking Water, Supplement III (Cincinnati, Ohio, USA, 1995).
Stephanie Kappes has been working as scientific writer and editor in the Marketing Department of Metrohm since 2013. She obtained her Bachelor degree in Nanosciences from the University of Basel (Switzerland). During her Master’s studies of Molecular and Cellular Life Sciences, which she completed at Utrecht University (The Netherlands), and several internships, she focused on the public communication of science.
Katinka Ruth works as product specialist in the Competence Centre for Ion Chromatography at Metrohm. She studied chemistry at the Technical University of Munich, Germany, where she obtained her Master's qualification with majors in organic chemistry and biotechnology. Katinka obtained her Ph.D. at the Swiss Federal Institute of Technology and the Swiss Federal Laboratories for Materials Science and Technology. Before joining Metrohm, she worked as a scientific assistant at the Swiss Federal Institute of Aquatic Science and Technology.
E-mail:ska@metrohm.comWebsite: www.metrohm.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










