Direct Determination of Cyanate in a Urea Solution and a Urea-Containing Protein Buffer
Urea is commonly used in protein purification, including large-scale purification of recombinant proteins for commercial purposes, and in recombinant protein manufacturing to denature and solubilize proteins (1). In aqueous solutions, urea degrades to cyanate and ammonium, with the maximum degradation rate occurring at neutral pHs commonly used in biological buffers (2). Cyanate is problematic in urea solutions because it carbamylates proteins, which causes unwanted modifications that can alter the protein's stability, function, and efficiency. Therefore, an accurate, sensitive method for determining cyanate in urea-containing buffers is required.
Urea is commonly used in protein purification, including large-scale purification of recombinant proteins for commercial purposes, and in recombinant protein manufacturing to denature and solubilize proteins (1). In aqueous solutions, urea degrades to cyanate and ammonium, with the maximum degradation rate occurring at neutral pHs commonly used in biological buffers (2). Cyanate is problematic in urea solutions because it carbamylates proteins, which causes unwanted modifications that can alter the protein's stability, function, and efficiency. Therefore, an accurate, sensitive method for determining cyanate in urea-containing buffers is required.
Ion chromatography can be used to determine cyanate in urea and urea-containing buffers (3). The authors used a Reagent-Free™ ion chromatography (RFIC™ ) system with a high- capacity column for this analysis. This RFIC method is sensitive, demonstrates good resolution of cyanate from a high concentration of chloride, and demonstrates good reproducibility.
Experimental Conditions
A Dionex RFIC system and AS Autosampler with cooling tray were used for this analysis. An IonPac® AS15 5-μm (3 × 150 mm) analytical column and guard (3 × 50 mm) were used with 25 mM KOH at 0.5 mL/min and 30°C to separate sample volumes of 5 μL. Cyanate was detected using suppressed conductivity with a Dionex ASRS® 300 suppressor in recycle mode at a current setting of 31 mA. Chromeleon® 6.8 chromatography software was used for system control and data processing. Column: IonPac® AG15 5-μm/ AS15 5-μm (3 mm); Eluent: 25 mM KOH; Eluent Source: EGC II KOH with CR-ATC; Temperature: 30°C; Autosampler Temperature: 4°C; Flow rate: 0.50 ml/min; Injection Volume: 5 μL; Detection: Suppressed conductivity with ASRS® 300; recycle mode, 31 mA.
Results
Hydroxide eluents produce significantly lower background conductivity, lower baseline noise, and therefore, lower detection limits than carbonate eluents. The IonPac AS15 column combined with electrolytically generated potassium hydroxide eluent yielded a detection limit of 0.25 μM cyanate. In freshly prepared samples containing 8 M urea and 8 M urea with 1 M chloride samples (diluted 50×) the cyanate concentrations were 1.1 to 1.3 μM (Figure 1A, 1C). The accuracy of cyanate was determined by calculating the recovery from the addition of cyanate at 1.2, 2.2 μM (Figure 1D), and 3.6 μM (Figure 1B) to the diluted 8 M urea containing samples. The calculated recoveries for the two sample sets were 97.1–104.4% for all solutions. The chromatograms demonstrate that cyanate elutes on the tail of the chloride peak tail and is well resolved from carbonate. This method can also be used to determine cyanate in urea solutions containing phosphate and sulfate buffers by adding a step change to 65 mM KOH at 12 min. More information on this application, including reproducibility data, can be found in Dionex Application Note 200. (4)
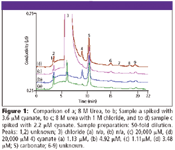
Figure 1
Using a high-capacity anion-exchange column in conjunction with suppressed conductivity detection, the authors accurately determined low (μM) concentrations of cyanate in solutions containing 8 M urea, as well as urea solutions containing molar concentrations of chloride. A Reagent-Free IC system ensures high precision and reproducibility, and eliminates the need to prepare mobile phase.
References
(1) Holtham, S.B.; Schütz, F. "The effect of cyanate on the stability of proteins." Biochim. Biophys. Acta, 1949, 3, 65–81.
(2) Hagel, P.; Gerding, J.J.T.; Fieggen, W.; Bloemendal, H. "Cyanate formation in solutions of urea. I. Calculation of cyanate concentrations at different temperature and pH." Biochim. Biophys. Acta, 1971, 243, 366–373.
(3) Lin, M-F.; Williams, C.; Murray, M.V.; Conn, G.; Ropp, P.A. "Ion chromatographic quantification of cyanate in urea solutions: estimation of the efficiency of cyanate scavengers for use in recombinant protein manufacturing." J. Chromatogr., B., 2004, 803(2), 353–362.
(4) Dionex Corporation. "Application Note 200 Direct Determination of Cyanate in Urea and Urea-Containing Protein Using a Reagent-Free Ion Chromatography," Dionex Corporation, Sunnyvale, CA. 2008.
Reagent-Free and RFIC are trademarks and IonPac, ASRS, and Chromeleon are registered trademarks of Dionex Corporation.
Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088-3603
tel. (408) 737-0700, fax (408) 730-9403

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis














