Determining Distribution of Erlotinib in Pancreatic Tumors by MALDI Using an LTQ Linear Ion Trap
Use imaging mass spectrometry to determine the biodistribution of erlotinib (Tarceva®) in pancreatic tumors from treated and untreated mice.
Use imaging mass spectrometry to determine the biodistribution of erlotinib (Tarceva®) in pancreatic tumors from treated and untreated mice.
Imaging Mass Spectrometry (IMS) using matrix-assisted laser desorption ionization (MALDI) is being investigated using a linear ion trap with the overall goal of analyzing the distribution of various targeted therapies within patients' tumors. The drug chosen for evaluation is erlotinib (Figure 1) which targets the epidermal growth factor receptor (EGFR), a type I receptor tyrosine kinase (TK) involved in cellular differentiation and proliferation, by binding to the ATP pocket and inhibiting the autophosphorylation of the receptor. Erlotinib has demonstrated clinical activity in nonsmall cell lung cancer, head and neck cancer, and ovarian cancer in Phase II studies. The sensitivity and MS-MS capabilities of the LTQ XL are exploited for the unambiguous determination of the distribution of this drug within human pancreatic tumors.
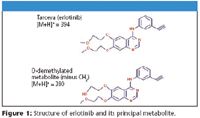
Figure 1
Methods
SCID mice bearing patients' pancreas tumors grown as xenografts were dosed once with 2.5 mg erlotinib in 6% Captisol, Captisol alone, or left untreated. Tissue was sectioned to 12 μm thick, placed on glass microscope slides and 6-aza-2-thiothymine (ATT) MALDI matrix applied with an airbrush.
Mass Spectrometry:
The laser was set to raster at 100 μm spacing and Xcalibur™ software was used for unattended data collection. Two and three dimensional maps were generated with ImageQuest™ 1.0 Software.
Results
Single Reaction Monitoring (SRM) experiments were used to monitor the m/z 278 fragment characteristic of both erlotinib (m/z 394) and its metabolite (m/z 380). This fragment ion appeared to be distributed evenly over the tumor. All expected erlotinib fragments (m/z 278, 304, 322, 336) were detected directly off tissue and images generated. Neither the drug nor the metabolite were found at significant levels in three untreated tissue sections that were analyzed with two different MALDI matrices (selection shown in Figure 2).
Figure 2
Conclusion
The LTQ with MALDI source makes for an outstanding platform for the study of drug distribution and its metabolites directly off tissue. The sensitivity of the linear ion trap, in particular, allows for an MS-MS spectrum with less than ten laser shots at each location.
MALDI as conducted in this study, without the use of isotopic labels or internal standards, is not considered a quantitative technique. Data processing for both treated and untreated tissues was kept consistent. A relative comparison of measurable drug between the two was therefore possible.
References
(1) Zhao, M, He, P. Rudek, M. A., Hidalgo, M., Baker, S. D. 2003. "Specific method for determination of OSI-774 and its metabolite OSI-420 in human plasma by using liquid chromatography-tandem mass spectrometry." J Chromatography B, 793, 413–420.

Thermo Fisher Scientific Inc.
355 River Oaks Parkway, San Jose, CA 95134-1991
tel. +1 800-532-4752; fax +1 561-688-8731

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis














