Determination of Seven Sodium Alkyl Sulphates in Environmental Water by Ion-pair Chromatography with Suppressed Conductivity Detection
LCGC Europe
A simple and fast ion-pair chromatography method to detect sodium alkyl sulphates in environmental samples using conductivity detection is described.
A simple and fast analytical method using ion-pair chromatography with suppressed conductivity detection was developed to simultaneously detect and quantify sodium decylsulphate (C10), sodium undecylsulphate (C11), sodium dodecylsulphate (C12), sodium tridecylsulphate (C13), sodium tetradecylsulphate (C14), sodium cetylsulphate (C16), and sodium octadecylsulphate (C18) in environmental water. The effect of the mobile phase parameters (NH4OH, CH3CN, and Na2CO3 concentrations) on the retention factors has been studied in detail, and the separation of the seven analytes was achieved using gradient elution.
Surfactants are widely used in personal and home care products including household detergents, shampoos, and cleaning products. They are also used in oil, pesticides, and metal processing (1–3). The annual worldwide consumption of surfactants is steadily increasing. Surfactants are classified into four groups according to their chemical characteristics: Cationic, anionic, non-ionic, and amphoteric (4). Anionic surfactants account for more than 40% of the total surfactants. Once in effluent sewage water, these anionic surfactants produce foams, emulsify, and suspend. They prevent the oxygen exchange between air and environmental water, resulting in the deterioration of water quality and potential harm to aquatic ecosystems (5–6). Recently, surfactants have been receiving more attention because of their growing occurrence in environmental water (7–8).

Several methods have been reported for the determination of anionic surfactants by spectrophotometry (9), liquid chromatography (LC) (10–12), and by electrochemical means (13). The spectrophotometry method is used for detections at low µg/L levels and suffers from chemical interferences. The LC method can detect the substances that contain a UV chromophore; and this method has been reviewed by Sohrabi (9). The surfactants which have to be derived can be detected by LC–UV. The electrochemical method can detect the compounds with oxidation and reduction. It is unsuitable for anion surfactants without oxidation and reduction potential.
This work opted for the suppressed conductivity detection based on the intrinsic properties of anion surfactants. Some papers have been published on ion chromatography investigations. Levine studied sodium dodecylsulphate in wastewater (14). Porter detected quantitatively poly disperse polyethylene oxide (PEO) and sodium dodecylsulphate (SDS) in water (15). In J. Weiss's publication, only five sodium alkyl sulphates have been detected (16). The focus of these studies were directed towards the traditional method and a limited number of analytes (17–18).
As a result, the aim of this work was to develop an ion chromatography method for the separation and precise quantification based on ion-pair chromatography with suppressed conductivity detection. This method offered three advantages: Firstly, it should be noted that the separation of seven species was achieved in less than 20 min. The effect of mobile phase parameters (NH4OH, CH3CN, and Na2CO3 concentration) and the optimization of a gradient elution on separation were studied. To the best of our knowledge, this is the first report on the separation of these seven analytes; secondly, the environmental water samples were injected into the ion chromatography instrument without any pretreatment; finally, suppressed conductivity is known to detect simple cationic, anionic compounds such as F or Cl-. Few ion-pair methods with suppressed conductivity detection for the separation of sodium alkyl sulphates are available. As a result of these advantages, this method can become a technique capable of the simultaneous determination of seven sodium alkyl sulphates in environmental water and can potentially be applied to environmental monitoring.
Experimental
Instrumentation: The ion chromatography (IC) system consisted of a 850 professional IC separation centre, a 872 gradient pump, a 771 IC compact interface, a 858 professional sample injector equipped with a 20 µL sample loop, and a suppressed conductivity detector (Metrohm). Conductivity suppression was performed by an anion chemical suppressor with a carbon dioxide suppressor (Metrohm). The chemical suppressor was operated in the external chemical mode, with 3 mM sulphuric acid as a regenerant. All instrument control and data collection was performed by MagIC Net 2.2 for Windows chromatography software (Metrohm).
The analytical column was a neutral polymer column, 4 mm × 250 mm, IonPacNS1 (Dionex). The resin in the columns was composed of ethyl vinyl benzene cross-linked polystyrene-divinylbenzene substrate (EVB-DVB) (Dionex).
Reagents: Sodium decylsulphate (C10), sodium undecylsulphate (C11), sodium dodecylsulphate (C12), sodium tridecylsulphate (C13), sodium tetradecylsulphate (C14), sodium cetylsulphate (C16), and sodium octadecylsulphate (C18) were obtained from Alorich (purity>99%).
HPLC-grade acetonitrile and analytical-grade ammonia were used (Merck). Deionized 18.2 MΩ water (Watsons) was used for eluent and standard solution preparation.
Preparation of Standard Solutions: Stock solutions and standard solutions for calibrations were prepared with deionized water. A mixed working solution at a concentration of 2500 mg/L was prepared by dissolving an accurately weighed quantity of 0.25 g of seven sodium alkyl sulphates into deionized water and diluting to 100 mL. The stock solution was serially diluted with deionized water to the concentrations of 200 mg/L, 150 mg/L, 100 mg/L, 50 mg/L, 30 mg/L, and 10 mg/L as the working solutions. They were kept in glass vials at 4 °C.
The Source of Samples: Samples of river water and sewage were collected from various locations in Lanzhou (China). The river samples were collected at surface level from discrete sections of the Yellow River in Lanzhou. Sewage samples were collected from living areas, industrial parks, and university areas. A total of 11 samples were obtained.
Chromatographic Separation: The separation column was a 4 mm × 250 mm, NS1 column (Dionex). A gradient elution was adopted by eluent A and eluent B. Eluent A was composed of a 5 mmol/L ammonium hydroxide +22% acetonitrile +0.3 mmol/L sodium carbonate; eluent B was 5 mmol/L ammonium hydroxide +80% acetonitrile. The following gradient programme was used throughout the experiment: 100% eluent A was increased to 100% B by a concave 2 mode at 20 min. The column oven was maintained at 30 °C. The sample injection volume was 20 µL. The flow rate was 1 mL/min. At 23 °C, the pH of eluent A and eluent B was 10.63 and 8.82, respectively. The chromatogram of an optimized separation of seven anions spiked in a 200 mg/L standard solution is shown in Figure 1.
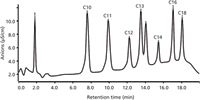
Figure 1: Optimized separation chromatogram of seven anions spiked in a 200 mg/L standard solution.
Results and Discussion
Ion-pair chromatography is based on the use of an apolar stationary phase, such as EVB-DVB polymers, which is dynamically modified by an ion-interaction reagent added to the mobile phase. Anions can be retained because of the formation of an electroneutral complex between the anionic sample and the ion-pairing reagent of the opposite charge (15,19). However, the mechanism of ion-exchange separation depends on the exchange of ions between a stationary phase and a mobile phase (20). According to the difference of retention in the stationary phase, the analytes can be separated from each other. Through ion-pair mechanism, the effects of following four parameters were investigated and are presented in this paper. These are organic solvents, ion-pairing reagents, inorganic additives, and gradient type selection.
Effect of Organic Solvents: Separation of the seven sodium alkyl sulphates is very difficult because of their similar physicochemical properties. In this study, the effects of organic solvents were to modify retention time and improve separation were investigated.
Common organic solvents are acetonitrile, methanol, and isopropanol. They make it difficult for surfactants to produce foams, and therefore achieve good reproducibility. The polarity of acetonitrile is lower than that of methanol. As a result, acetonitrile was added to reduce the mobile phase polarity. Isopropanol has a higher viscosity, leading to higher column pressure and probably lower column efficiency (21). Acetonitrile can obtain a better separation and has a shorter retention time in comparison with methanol and isopropanol. Acetonitrile was therefore adopted as an organic solvent.
Figure 2 shows the effect of acetonitrile in a range of concentrations from 15%, 18%, 20%, 22%, and 30% (v/v) on the retention time for the analytes. The increase of acetonitrile from 15% to 22% in the eluent can reduce retention times of the seven sodium alkyl sulphates. For C10–C14 sodium alkyl sulphates, acetonitrile at a concentration of 22% to 30% (v/v) had less effect on their retention times. Longer alkyl chains of sulphates had stronger hydrophobic interactions. It was observed that the retention time was at an optimum when adding 22% (v/v) acetonitrile to the mobile phase for the separation.
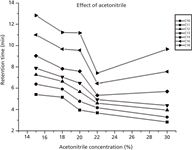
Figure 2: Effect of acetonitrile concentration on retention time for the analytes studied. Column: IonPac NS1 (4 Ã 250 mm). Eluent B: 80% acetonitrile. Eluent A: acetonitrile as shown.
Effect of Ion-pairing Reagents: The most commonly used ion-pairing reagents presented a large hydrophobic moiety and a small ionized group whose charge is opposite to that of the samples. The effect on the retention of ionic samples is interpreted by the adsorption of the ion-interaction reagent on the hydrophobic surface, leading consecutively to the formation of charged sites. Ionic samples are then retained by electrostatic forces in the layer adjacent to the surface of the particles in the stationary phase (15). Owing to the presence of ion-pair reagents, sample ions can be exchanged and retained on the consecutive ion-exchange surface of the stationary phase.
Hydrophilic ion-pairing reagents are suitable for hydrophobic analytes separation. The reagents of smaller molecular mass have more effect on separation than larger molecular mass. Ammonium hydroxide is the strongest hydrophilic and has the smallest molecular mass in comparison with other ion-pairing reagents such as tetrabutyl ammonium hydroxide (TBAOH). Ammonium hydroxide is the preferred ion pairing reagent for analysis of high molecular masses and long alkyl chains such as sodium alkyl sulphates.
This study contrasted three concentrations with one another. The results showed that the effect on retention time was less significant with a change in the NH4OH concentration range 4–10 mmol/L. Seven analytes were not completely separated and had longer retention times at 4 mmol/L. Both 5 mmol/L and 10 mmol/L NH4OH obtained complete separation. However, the peak height of each component reached the maximum value when 5 mmol/L NH4OH was used for the determination of sodium alkyl sulphates. It obtained an efficient separation, with sharp and symmetrical peaks (Figure 3).

Figure 3: Effect of NH4OH concentration on retention time for the analytes studied. Column: IonPac NS1 (4 Ã 250 mm). Eluent A: 22% acetonitrile; eluent B: 80% acetonitrile. The concentration of chromatogram (a): 4 mmol/L; (b): 5 mmol/L; (c): 10 mmol/L NH4OH.
Effect of Inorganic Additives: The effect of organic solvents was evaluated at 22% (v/v) acetonitrile and 5 mmol/L ammonium hydroxide. Alkyl sodium sulphates are two valences, long alkyl chains, and strong hydrophobic anions. Because of the selectivity exhibited by the analytical column, a Na2CO3 eluent was initially chosen to improve separation and selectivity.
The team studied the effect of concentration of Na2CO3 at a concentration of 0.1 mmol/L, 0.3 mmol/L, and 0.5 mmol/L. The results showed the influence of a small amount of Na2CO3 on C10–C14 sodium alkyl sulphates. It was observed that retention time increased as the inorganic additives concentration of the analytes was increased. It was 0.3 mmol/L Na2CO3 that achieved optimum retention times and a complete separation.
Eluent Gradient Type Selection: The number of the analytes and their similar structure made the optimization of the separation a difficult task. If a low concentration of leachate is chosen, the seven sodium alkyl sulphates cannot be separated thoroughly in 20 min. Strongly reserved substances will have a long retention time. From the experiments, it was found to be necessary to optimize the eluent composition gradients. There are nine kinds of gradient types: linear; concave 1; concave 2; concave 3; concave 4; convexity 1; convexity 2; convexity 3; and convexity 4.
The acetonitrile content ranged from 22% to 80% in 20 min. Linear gradient was not able to separate the C10–C14 sodium alkyl sulphates well and caused broad peaks, with the result that the elution power was significantly decreased (concave 1) in a shorter period of time. In subsequent experiments, concave 2, concave 3, and concave 4 gradients were tested. The gradient of concave 3 had broad peaks. C12–C18 sodium alkyl sulphates had longer retention times when concave 4 was used. However, the concave 2 gradient ensured that all the compounds separated thoroughly and achieved sharp and symmetrical peaks. As a result, a gradient type of concave 2 was chosen (Figure 4).

Figure 4: Effect of different gradient types on retention time for the analytes studied. Column: IonPac NS1(4 Ã 250 mm). (a): Linear; (b): Concave 1; (c): Concave 2; (d): Concave 3; (e): Concave 4.
Linear Ranges and Detection Limits: The linear range was calculated with the slope variation. Different concentrations of working standard solutions from 10 mg/L to 200 mg/L were injected. The results showed that the seven sodium sulphates had good linear regression and a wide linear range. Detection limits were evaluated at three times the background signal and were determined injecting 20 µL of working standard solutions. The detection limits were 10 mg/L for the seven sodium alkyl sulphates. The results are shown in Table 1.
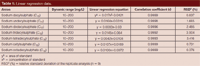
Table 1: Linear regression data.
Repeatability and Reproducibility: The repeatability of the peak areas and the retention times for the seven sodium alkyl sulphates were studied by repeatedly injecting nine replicate analyses of mixed 10 mg/L standard solutions. It was observed that both retention times and peak areas were reproducible and all the relative standard deviation (RSD) (n = 9) values obtained were satisfactory (<4.0%). The results are shown in Table 1.
To calculate the reproducibility conveniently, the study selected the Yellow River in Lanzhou (China) without sodium sulphates as the blank sample. The RSD (n = 9) spiked with three concentrations of 30 mg/L, 50 mg/L, and 100 mg/L into the blank sample were in the range 0.62–4.24%. The analytical procedure showed good precision and accuracy for the determination of the standard solution in different concentrations. The results are shown in the Table 2.

Table 2: Relative standard deviations and recovery results when adding different concentrations.
Real Sample Analysis and Recoveries: After optimization, the ion-pair chromatography method was applied to the simultaneous determination of the seven sodium alkyl sulphates in the river samples from the Yellow River in Lanzhou (China) and the sewage samples from living areas, industrial parks, and university areas.
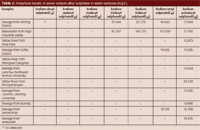
Table 3: Analytical results of seven sodium alkyl sulphates in water samples (mg/L).
The work also selected the Yellow River without alkyl sodium sulphates as the blank sample. These samples have been divided into three groups which have been spiked with 30 mg/L, 50 mg/L, and 100 mg/L of a mixed standard solution of seven sodium sulphates, respectively. These were measured in accordance with the optimization of the experimental method and compared to a blank experiment. Recoveries of 92.8–100.5% (n = 3) were obtained. The results of analytical samples are shown in Table 3. The chromatogram of the real sample from the Xigu (Lanzhou, China) industrial estate is displayed in Figure 5.
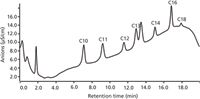
Figure 5: Chromatogram of a real sample in Xigu (Lanzhou, China) industrial estate.
The chromatograms achieved a good application to real sample analysis without time-consuming pretreatment. The results demonstrated that industrial wastewater had the higher concentration of sodium tridecylsulphate (C13), sodium tetradecylsulphate (C14), sodium cetylsulphate (C16), and sodium octadecylsulphate (C18). Most sewage samples contained octadecylsulphate (C18).
Conclusions
Separation was achieved after a study of the effect of mobile phase parameters (NH4OH, CH3CN, and Na2CO3 concentration) and an optimization of gradient elution. The application of ion-pair chromatography in analyzing polar organic compounds was able to make up the deficiencies of LC. It should be noted that suppressed conductivity was coupled to an ion-pair mechanism, and the technique was developed for the determination of seven sodium alkyl sulphates. The average recoveries for samples were in the range of 92.8% to 100.5% (n = 3) and the RSD (n = 9) was less than 4.24%. The method can therefore be applied for the analysis of numerous surfactant materials such as sodium decylsulphate (C10), sodium undecylsulphate (C11), sodium dodecylsulphate (C12), sodium tridecylsulphate (C13), sodium tetradecylsulphate (C14), sodium cetylsulphate (C16), and sodium octadecylsulphate (C18) in environmental water.
Yuan Chun-li is currently studying for a PhD at the School of Mechanical Engineering and Automation, Northeastern University, Shenyang, China.
Zhou Wei is the director of the Central Laboratory of Technical Center of Gansu Entry-Exit Inspection and Quarantine Bureau, Lanzhou, China. He sits on the CIQ Technical Committee of Food Inspection Professional, and the council in Chinese Society of Fourth Chromatography. He is also a professor at the Northwest Normal University and Gansu Agricultural University.
Zhu Tong is a professor at the School of Mechanical Engineering and Automation, Northeastern University, Shenyang, China. He is the director of the Process Equipment and Environmental Engineering Research Institute. He is also a member of the International Water Association; the China Environmental Science Society; and the Japan Society of Water Environment.
Wang Bo works in the Central Laboratory of Technical Center of Gansu Entry-Exit Inspection and Quarantine Bureau in Lanzhou, China.
References
(1) Bo Gao and Mukul M. Sharma, J. Colloid Interface Sci.404(15), 80–84 (2013).
(2) S. Tai, Z. Gao, X. Liu, and Q. Zhang, Eru. J. Lipid Sci. Technol.114(9), 1062 (2012).
(3) T.M Schmitt, Analysis of Surfactants, 2nd ed. (M. Dekker Press, New York, USA, 2001), pp. 194.
(4) H.R. Ryu, H.S. Park, and C.K.Rhee, Bull Korean Chem. Soc.28(1), 85–88 (2007).
(5) J.Blasco, E.Gonzalez, and C. Sarasquete, Toxicol Environ. Chem.71 , 447–456 (1999).
(6) Yu Lingyun, Sun Dongmei and Zhang Xinshen, Lether science and engineering 19(2), 72–76 (2009).
(7) O.V. Stepanets, G.Y. Solov, A.M. Mikhailova, and A.I. Kulapin, J. Anal. Chem.56(3), 290–293 (2001).
(8) N. Márqueza, B. Bravo, F. Ysambertt, G. Chávez, N. Subero, and J.L. Salager, Anal. Chim. Acta477(2), 293–303 (2003).
(9) M.R. Sohrabi, N.A. Hassan, A. Adnani, and M.H. Fard, J. Appl. Sci.7, 148–150 (2007).
(10) H.S. Park, H.R. Ryu, and C.K. Rhee, Talanta70(3), 481–485 (2006).
(11) S. Cheng, B. Margot, A. Hamish, D. Anderson, and L. Brydon, J. Chromatogr. A771(1–2), 145–154 (1997).
(12) K. Rissler, J. Chromatogr. A 74(2), 21–54 (1996).
(13) J. Sanchez and M. Valle, Talanta54(5), 893–902 (2001).
(14) L. Levine, J. Judkins, and J. Garland, J. Chromatogr. A874(2), 207–215 (2000).
(15) F.I. Portet, C. Treiner, and P.L. Desbene, J. Chromatogr. A878(1), 99–113 (2000).
(16) J. Weiss, Handbook of Ion Chromatography, 3rd ed. (Wiley-VCH Verlag GmbH &Co. KgaA. 2005), pp. 393–444.
(17) M.C. Bruzzoniti, R.M. Carlo, and C. Sarzanini, Talanta75(3), 734–739 (2008).
(18) C.L. Guan, J. Ouyang, Q.L. Li, B.H. Liu, and W.R.G. Baeyens, Talanta50(6), 1197–1203 (2000).
(19) C. Horvath, W. Melander, I. Molnar, and P. Molnar, Anal. Chem.49, 2295–2305 (1977).
(20) P. Hajos and G. Revesz, J. Chromatogr. A771(1–2), 23–33 (1997).
(21) Shifen Mu, Methods and applications of ion chromatography, 2nd ed. (Chemical Industry Press, China, 2005), pp. 124.

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.









