Determination of Fipronil and Its Metabolites in Eggs and Environmental Matrices by LC–MS/MS
Fipronil is a widely used insecticide. Exposure of humans to fipronil can cause harmful health effects. The aim of the present work is to develop a liquid chromatography–tandem mass spectrometry (LC–MS/MS) method for the rapid detection of fipronil and its metabolites in eggs and environmental matrices (soil, feed, and chicken feed bags). Solvent extraction was performed with a mixture including water, acetonitrile, and sodium chloride, followed by purification using a solid-phase extraction column. The LC separation was accomplished on a C18 column, and the detection was performed with a triple quadrupole mass spectrometer using electrospray ionization in multiple reaction monitoring (MRM) mode. The results showed a good linear relationship between fipronil and its metabolites in the range of 0.01 to 20 ng/mL, and the linear correlation coefficients (R2) were all greater than 0.999. The detection and quantification limits of the method for all analytes were 0.01 and 0.05 ng/mL, respectively. The precision expressed as coefficients of variation (n = 3) for assay of fipronil and its metabolites ranged from 8.4% to 13.2% at three levels (0.1 ng/mL, 1 ng/mL, and 10 ng/mL). The recoveries ranged from 81.3% to ~119.5%. Finally, we successfully employed the method to a case of fipronil contamination, and found residues of fipronil and its metabolites in eggs and the environmental matrix.
Fipronil is a type of benzopyrazole insecticide developed in 1987 by Bayer Scientific Crops Co. Ltd. (1), and is widely used to control crop pests in vegetables, rice, tobacco, cotton, animal husbandry, and public health. Fipronil is metabolized slowly in the environment, and has a half-life of about 125 days (2). Exposure to fipronil causes negative health effects to humans, such as skin irritation, sweating, nausea, vomiting, headache, stomach pain, dizziness, weakness, and seizures. It was reported in 2017 that eggs contaminated with fipronil had been widely found all over the Europe (3).
In China, the abuse of fipronil is also widespread. A recent case occurred at a farm in Beijing where fipronil was heavily used as a pesticide in chicken feeding. The National Center of Biomedical Analysis was asked to help provide data concerning fipronil and its metabolites for the eggs of chickens at this farm and environmental matrices (including soil, chicken feed, and chicken feed bags). To achieve this purpose, it was essential to establish a rapid and sensitive method for identification and quantification of fipronil and its metabolites in different matrices.
In the existing literature, the detection of fipronil in honey, soil, and water (4–7) has been reported, and the detection methods were focused on analytical techniques such as gas chromatography (GC), GC–tandem mass spectrometry (GC–MS/MS), liquid chromatography–tandem mass spectrometry (LC–MS/MS), and high performance liquid chromatography (HPLC) (8–10). LC–MS/MS, because of its excellent sensitivity, ensures the minimum detection limit of ultratrace materials, and has high performance and reliability standards; the technique is widely used in various fields. Recently, Li Xiaoming (11) developed a simple and sensitive method for the analysis of fipronil residues in animal-derived foods using LC–MS/MS, but he did not evaluate other types of matrices. There are few reports of simultaneous detection of fipronil and its metabolites in multiple types of matrices by LC–MS/MS, especially in fipronil product packaging, which is similar to packaging of beverages (leading to cases of poisoning) (12).
In this study, we describe a method for the detection of fipronil and its metabolites (Figure 1) in eggs and environmental matrices based on solvent and solid-phase extraction and LC–MS/MS. We hope that our study can solve the problem of trace analysis of fipronil and its metabolites in various matrices.
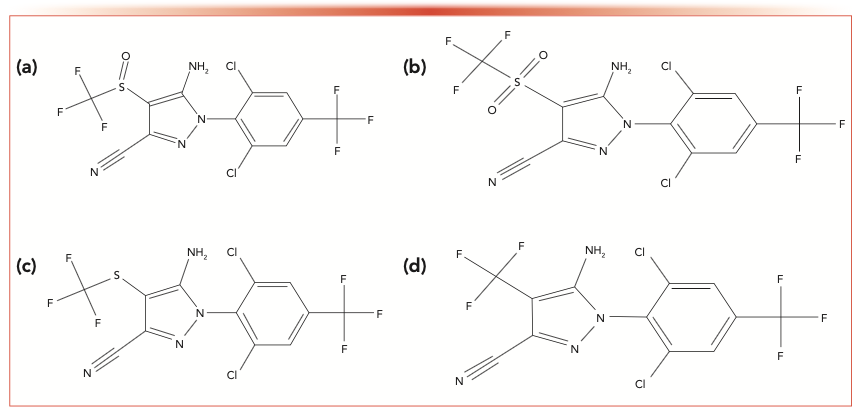
FIGURE 1: Molecular structures of (a) fipronil, (b) fipronilsulfone, (c) fipronilsulfoxide, and (d) fipronildesulfinyl
Materials and Methods
Instruments
A Waters Xevo TQ-S triple-quadrupole mass spectrometer, equipped with an electrospray ionization source, was used. A Waters Acquity ultrahigh performance liquid chromatography (UHPLC) system was used to perform LC analysis. The turbine mixer was from Shanghai Jingke Industrial Co. Ltd. The electronic balance was a product of O’Haus Instruments Co. Ltd., and the high speed freezing centrifuge is manufactured by Sigma-Aldrich.
Regents and Materials
Eggs, soil, and chicken feed bags were acquired from a chicken farm in Beijing, China. NaCl was product of China Pharmaceutical Group Chemical Reagent Co., Ltd. Acetonitrile and acetic acid were chromatographically pure, produced by Merck. A column extractor (60 mg/3 mL) and nylon needle filter were produced by Agela. Fipronil and its metabolites (fipronil, fipronil desulfinyl, fipronil sulfone, and fipronil sulfoxide) were purchased from the National Standard Centre.
Preparation of Stock and Working Solutions
Individual stock solutions of fipronil, fipronil desulfinyl, fipronil sulfone, and fipronil sulfoxide were prepared in a concentration of 200 μg/mL by dissolving 2 mg of each compound in 10 mL acetonitrile. All stock solutions were stored at -20 °C in the dark until use. Each stock solution was mixed and diluted to concentration of 1000 ng/mL in acetonitril as mixed stock solution. The working solutions were prepared by freshly diluting the mixed stock solutions with acetonitrile at concentrations of 100, 50, 20, 10, 1, 0.5, 0.1, 0.05, and 0.01 ng/mL, respectively. All standard working solutions were also stored at –20 °C in the dark until use.
Extraction and Purification
Each sample (5 g) was homogenixed in a blender, and transferred to a 15 mL centrifuge tube, 5 mL water was added, followed by 10 mL acetonitrile containing 1% acetic acid (v/v). The mixture was briefly shaken and extracted by ultrasound for 10 min. Then, 3 g of NaCl was added to the mixture. After centrifugation for 5 min at 8000 RPM/min, the supernatant (2 mL) was transferred to an extraction column (no activation or leaching required). The first 0.5 mL outflow was discarded, and the subsequent sample loading outflow was collected. Next, 0.5 mL water was added to the 0.5 mL of collection solution, and filtered with a 0.22 μm nylon filter. The filtrate was collected and stored at –20 °C in the dark for later use.
LC Analysis
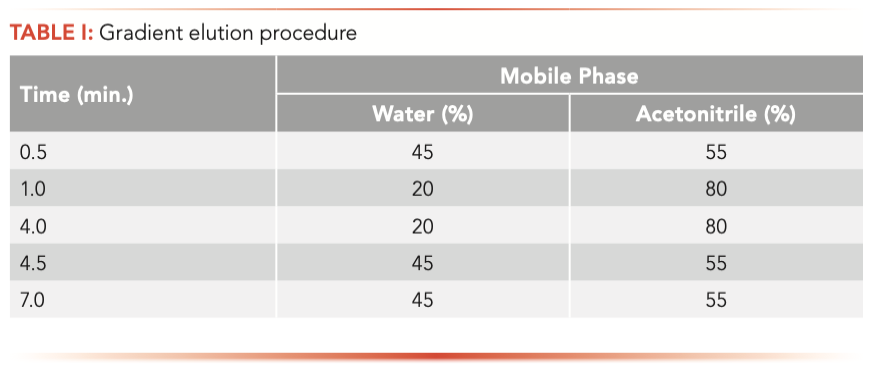
LC analysis was performed with a Waters Acquity UHPLC system. The analytes were separated on an Agela Venusil MP C18 column (3.0 x 50 mm, 3 μm) in gradient elution mode (Table I) with water–acetonitrile as mobile phase. The column temperature was maintained at 30 °C during LC analysis; the injection volume was 5 μL, and a flow rate of 0.4 mL/min was used.
Tandem Mass Spectrometry
The mass analysis was performed with a triple quadrupole mass spectrometer equipped with an electrospray ionization source. Waters Masslynx 4.0 software was used to control the spectrometer, and to collect the data. The electrospray ionization (ESI) source was operated in the negative-ion mode, and the conditions were set up as following: Electrical spray voltage: –4500 V; aerosol pressure: 45 psi; air curtain pressure: 15 psi; auxiliary gas pressure: 50 psi; ion source temperature: 450 °C. The ions were monitored in multiple reaction monitoring (MRM) mode.
Results and Discussion
MS/MS Detection
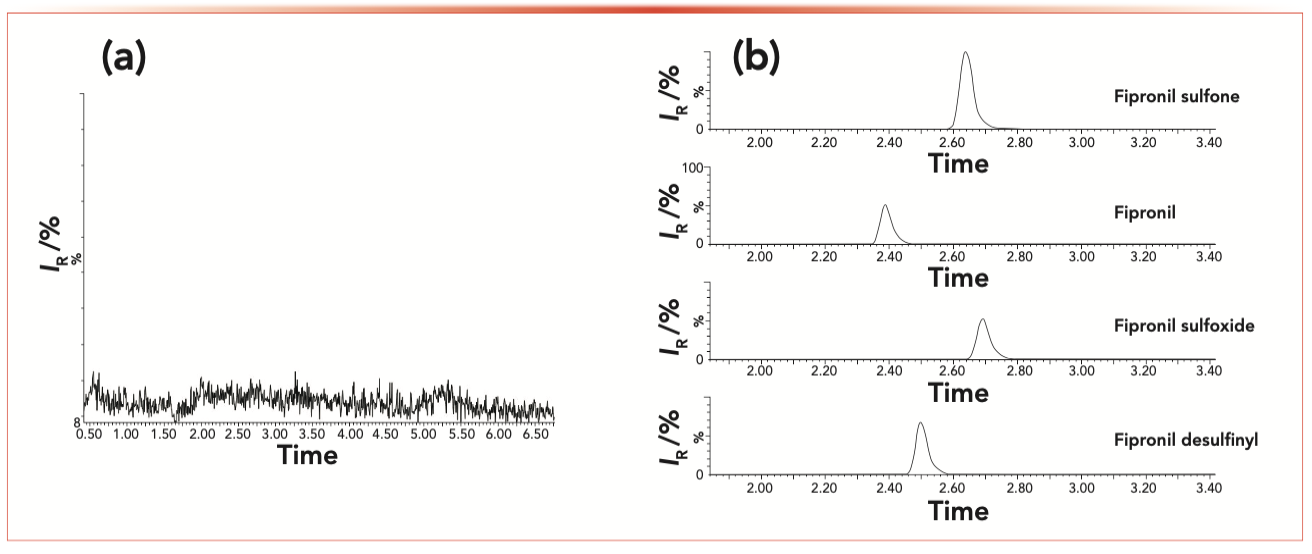
Each reference solution was directly injected into the mass spectrometer with a concentration of 0.5 mg/L. In MS tuning mode, four parent ions are tuned manually. At this stage, the positive and negative ion modes were used to compare their sensitivity to analyte molecular ions. It was found that, in ESI mode, all four samples had higher responses. The optimized parameters are shown in Table II. The MRM chromatograms for the blank control and fipronil and its metabolites are shown in Figure 2.
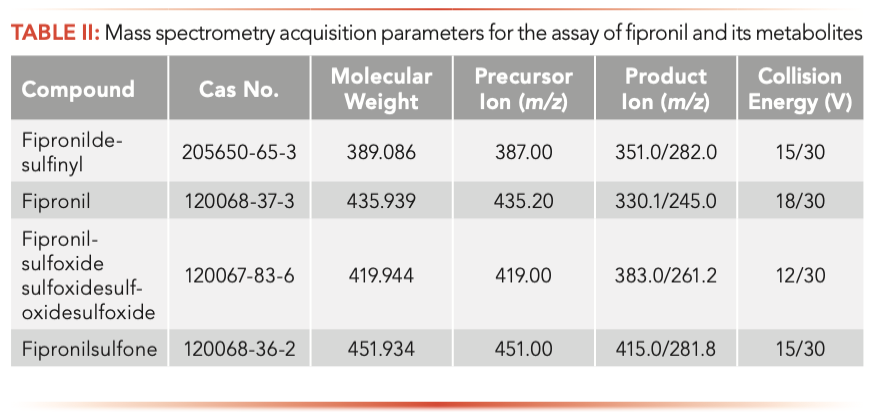
FIGURE 2: Typical MRM chromatogram for (a) blank chicken eggs, and (b) analytes each spiked at the concentration of 0.1 ng/mL.
Method Validation
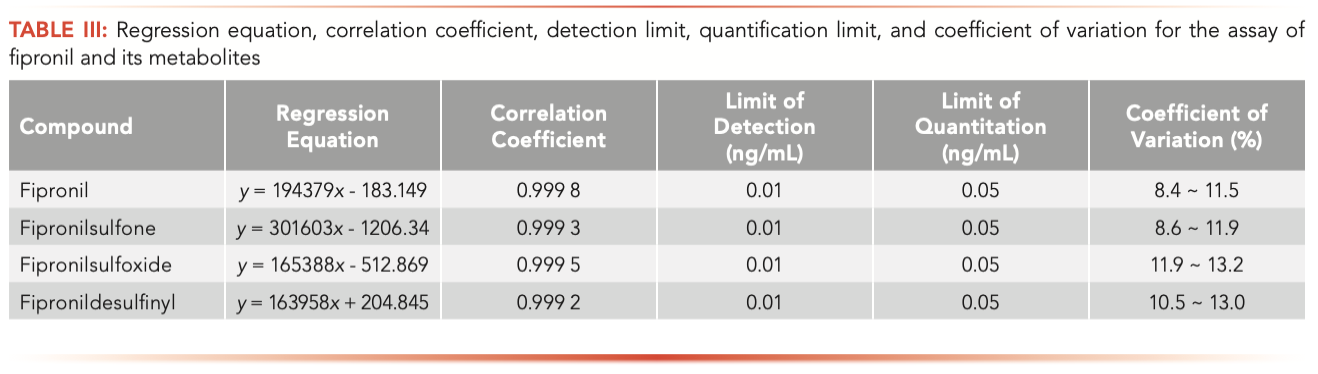
The results of the linearity evaluations for fipronil, fipronil desulfinyl, fipronilsulfone, and fipronil sulfoxide are summarized in Table III. Calibration curves showed good linearity for all compounds in the concentration range of 0.05~20.0 ng/mL, and the linear correlation coefficients (R2) were all greater than 0.999. The detection limit of fipronil and its metabolites were all 0.01 ng/mL (estimated based on the signal-to-noise ratios [S/N] 3:1), while the determined quantitative limit were all 0.05 ng/mL with the S/N of 10:1. This method shows the potential to detect trace amounts of fipronil and its metabolites in various matrices. Under the abovementioned test conditions, the samples of fipronil and its metabolites were prepared at three concentrations (0.1 ng/mL, 1 ng/mL, and 10 ng/mL), and each was continuously tested six times for precision estimation. The coefficient of variation (CV) for the assays of fipronil and its metabolites, calculated based on six replicate analyses, were between 8.4~13.2%, indicating that the method has good precision. The results are shown in Table III.
Recoveries and Matrix Effects
Recovery and matrix effect tests were performed with spiked samples. Fipronil and its metabolites were added to the soil, plastic bags, and eggs at the final concentrations of 0.1 ng/mL, 1 ng/mL, and 10 ng/mL, respectively. Recovery experiments were performed by comparing the analytical results of the extracted samples with unextracted standards (in acetonitrile for this test) representing 100% recovery. The recoveries for the assay of fipronil and its metabolites ranged from 81.3% to 119.5% in three different matrices. The matrix effects of all the compounds were evaluated by comparing the MS/MS responses of fibufenil and its metabolites at a concentration of 0.1 ng/mL in six batches of blank soil, plastic bags, and egg extract with those of the analytes with acetonitrile. The matrix effects for all the analytes were not significant. This method has good anti-interference ability, and can be used for the determination of trace fipronil and its metabolites under various conditions.
Method Application to Authentic Case
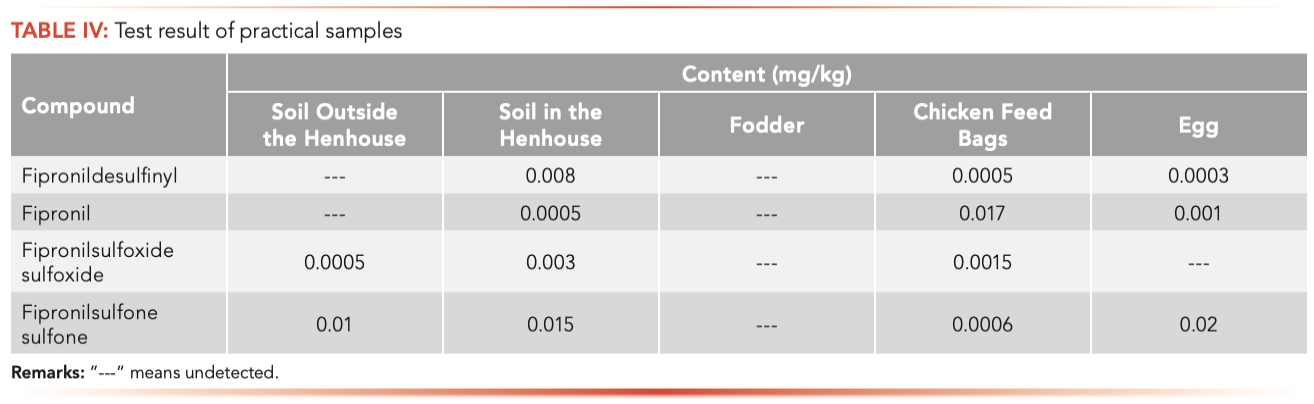
After the outbreak of fipronil contamination in Europe, we received a request from a large farm in Beijing for the detection of fipronil and its metabolites. The samples include the eggs, soil in and outside the henhouse, chicken feed, and chicken feed bags. All samples were extracted with a mixture including water, acetonitrile, and sodium chloride, and then purified using a solid-phase extraction (SPE) column (PEP Plus, Agela Technologies). The extraction was analyzed by LC–MS with the method described here. The test results are shown in Table IV. Fipronil and its metabolites were all detected in soil in the henhouse. Fipronil and two metabolites were detected in eggs, but fipronil sulfoxide was not detected. Two metabolites were detected in the soil outside the henhouse, but no fipronil or fipronil desulfinyl were detected, probably because fipronil had been degraded. Surprisingly, fipronil and its metabolites were not detected in chicken feed, but they were detected in chicken feed packaging, prob- ably because only the soil was contaminated, therefore there was only adhesion to the outside of packaging, but the feed was not contaminated. Tests showed that eggs and the environment at the farm had been contaminated with fipronil, and eggs from the farm were advised against consumption.
Conclusion
In this study, an LC–MS/MS method has been established for the detection of fipronil and its metabolites in eggs and the chicken farm environment. Solvent extraction coupled with SPE were used for pretreatment, which has high extraction efficiency and simple and quick operation. The whole analysis process is simple and fast. The method has good accuracy and high precision, and is suitable for the trace analysis of fipronil and its metabolites in soil, organic materials, and food samples. Finally, we successfully employed the method in a case of fipronil contamination.
Acknowledgment
This work was supported by National Key R&D Program of China (2017YFC1601100;2017YFC1601101).
References
(1) C.C.D. Tingle, J.A. Rother, C.F. Dewhurst, et al., Rev. Environ. Contam. Toxicol. 176, 1–66 (2003).
(2) J.M. Bonmatin, C. Giorio, V. Girolami, et al. Environ. Sci. Pollut. R 22(1), 35–67 (2015).
(3) H. Xiao-Fei, W. Feng-Xian, and L. Xiang-Li, J. Food Saf. & Qual. 9(6), 1226–1233 (2018).
(4) J.J Jiménez, J.L. Bernal, and M.J.D. Nozal, J. Chromatogr. A 1187(1–2), 40–45 (2008).
(5) Y. Cheng, F. Dong, and X. Liu, Anal. Methods 6(6), 1788 (2014).
(6) M. Paramasivam and S. Chandrasekaran, Int. J. Environ. An. Ch. 93(11–15), 1203–1211 (2013).
(7) A.L.D. Toffoli, K.D. Mata, and M.C. Bisinoti, J. Environ. Sci. Heal. B 50(10), 753–759 (2015).
(8) K.S.R. Raju, I. Taneja, and M. Rashid, Sci. Rep. 6, 22447 (2016).
(9) Y. Zhang, Y.G. Zhao, and H. Cheng, Anal. Methods 10, 1673–1679 (2018).
(10) S. Biswas, R. Mondal, A. Mukherjee, M. Sarkar, and R.K. Koleb, Food Chem. 272, 559–567 (2018).
(11) X.P. Li, X.Q. Liang, and N. Cheng, Anhui Agr. Sci. 47(14), 206–234 (2019).
(12) F. Mohamed, L. Senarathna, and A. Percy, Toxicol. Clin. Toxicol. 42(7), 955–963 (2004).
Shengming Wu, Junqing Zhao, Junjian Fang, Hui Li, and Fangting Dong are with the National Center of Biomedical Analysis, in Beijing, China. Direct correspondence to: dft@proteomics.cn

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.










