Determination of Iodide and Iodate in Seawater by HPLC with UV Detection
Iodine is an essential nutrient in seawater, seafood, and iodine-enriched foods, such as iodized table salt. The most common forms of iodine in the diet are iodide and iodate, with additional iodo-organic compounds providing a small fraction of the bio-available iodine. Iodine deficiency affects thyroid hormone production and leads to developmental diseases, goiter, and paralysis (1).
Iodine is an essential nutrient in seawater, seafood, and iodine-enriched foods, such as iodized table salt. The most common forms of iodine in the diet are iodide and iodate, with additional iodo-organic compounds providing a small fraction of the bio-available iodine. Iodine deficiency affects thyroid hormone production and leads to developmental diseases, goiter, and paralysis (1).
Because iodide and iodate are essential sources of iodine, these anions must be determined in a variety of matrices. In seawater, for example, iodide amounts can range from less than 1 μg/L to greater than 60 μg/L with the measured concentrations dependent on water depth, oxygen concentration, and the biological mediation of the iodide/iodate equilibrium (2). Determination of iodide and iodate in saline matrixes is challenging due to the high chloride concentrations in samples. In seawater, the matrix is further complicated by high concentrations of carbonate and sulfate. This high ionic strength matrix makes analysis of samples containing iodide and iodate difficult.
The analysis method described here is specific, sensitive, and rapid. Iodide and iodate are separated on the Acclaim® Mixed-Mode WAX-1, a silica-based column that incorporates hydrophobic, weak anion-exchange, and ion exclusion properties. Consequently, retention of basic, neutral, and acidic molecules can be independently or concurrently adjusted by changing mobile phase ionic strength, pH, or organic solvent content. The ability to control these properties leads to unique and adjustable selectivity. This column effectively separates anions with the high capacity necessary for the analysis of iodide and iodate in saline matrices, minimizing the need for sample pretreatment.
Experimental
A Dionex UltiMate® 3000 system with a HPG-3200M pump, a TCC-3200 column compartment, a WPS-3000TSL Micro autosampler, and a PDA-3000 detector were used. An Acclaim Mixed-Mode WAX-1, 5 μm 2.1 × 150 mm column was used for all separations with the conditions in Figure 1. Chromeleon® Chromatography Management Software was used for system control and data processing.

Figure 1
Results and Discussion
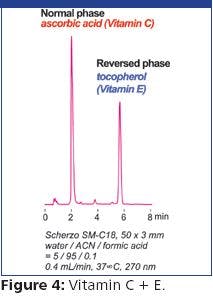
Figure 1 shows the separation of iodate and iodide in a 1:10 dilution of synthetic seawater on the Acclaim Mixed-Mode WAX-1 column. A wavelength of 223 nm was determined by collecting data between 210–500 nm and choosing the wavelength with the best signal to noise (S/N) ratio for iodide. Iodide and iodate can be determined simultaneously in less than 15 min. Iodide is well separated from all other peaks and iodate is easily identified. Although this sample was diluted to determine iodate, no sample dilution is necessary for iodide determination.
Linear response to iodide and iodate was confirmed between 10–250 and 500–7600 μg/mL (ppb), respectively. The standards were prepared in deionized (DI) water without the added complexity of matching the sample matrix. Determinations of iodide and iodate in saline matrixes were accurate and precise with recoveries ranging between 93–113% and peak area RSDs +/- 1.2%. The data shows the advantage of the high capacity and adjustable specificity of the Acclaim Mixed-Mode WAX-1 column to determine analytes in high ionic strength samples.
References
(1) Iodine Status Worldwide: WHO Global Database on Iodine Deficiency, de Benoist, B.; Anderson, M.; Egli, I.; Takkouche, B.; Allen, H.; eds. Department of Nutrition for Health and Development, World Health Organization, Geneva, 2004.
(2) Tian, R.C.; Nicolas, E.; Iodide Speciation in the Northwestern Mediterranean Sea: Method and Vertical Profile, Marine Chemistry, 1995, 48, 151.
Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088-3603
tel. (408)737-0700, fax (408)730-9403
Website: www.dionex.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)