Defining Material Used in Biopharmaceutical Analysis
There is a need to use more descriptive and precise terms to describe some of the properties of the products used for biopharmaceutical analysis. It is probably more correct to say “low adsorption” and “corrosion-resistant” systems.
People often ask, what do “bioinert”,” biocompatible”, “metal-free”, and “inert” actually mean and what is the difference between them—if there is any? The terms “stainless steel‑free”, “truly inert”, or “fully biocompatible” are also used in this sense.
The first mention of the term “bioinert” dates back to 1933 and was related to the use of alumina as a material for biomedical applications (1). Since then, there has been significant interest in continually developing novel materials for biomedical implants. The term “bioinert” has since diffused into many other fields and different contexts. One of these fields is liquid chromatography.
When separating biomolecules, chromatographers often encounter unwanted and unpredictable interactions between samples and chromatographic surfaces (injector, tubes, column hardware, and detector cells) (2). Indeed, adsorptive interactions negatively affect chromatographic performance by altering peak shape (asymmetry, tailing) and reducing the recovery (3).
What is certain is that there are two different concerns. It is important to mention that both issues are associated with the chromatographic hardware (LC system and column) and not with the stationary phase (though in some similar ways, stationary phases must also be carefully designed). One issue with chromatography hardware is the potential adsorption of solute molecules onto wetted surfaces (nonspecific adsorption) in an LC instrument (sample flow path) or within the column hardware, especially on frits and the inner wall of the column tubing. It is most appropriate to call any technologies and materials that improve analyte recoveries and limit undesired interactions with the hardware as being bioinert. To be more precise with the terminology, we could refer to these as low adsorption materials. The other concern with chromatography hardware is related to the resistance of the system and its columns to harsh conditions typically applied for biomolecule separations. The use of halide mobile phase components, for example, chloride ions, can corrode stainless steel surfaces, and this is one of the clearest examples where chemical resistance is needed. A chromatographic system designed to be resistant to such conditions is often termed as being biocompatible.
In nondenaturing modes of chromatography, mobile phases often contain high concentration of salts (for example, 0.2–2 M). Stainless steel can, and does, rust and corrode if continuously exposed to high concentrations of halide mobile phase salts. The corroded metal can form metal complexes that leach onto the column, interact with the stationary phase, clog the frits, and negatively affect peak shape and resolution. Stainless steel may also release iron ions into the mobile phase, and these ions can bind to the stationary phase and interact with metal‑sensitive analytes. On the other hand, in denaturing LC conditions, low pH (pH 2–3) mobile phases are frequently applied and the mobile phase temperature is often set to 70–90 °C, which can also be an extreme condition for the hardware to withstand. Therefore, in a biocompatible system, alternative materials are needed, for example, PEEK, sapphire, titanium, or specialized metal alloys. In addition, pumps and autosamplers need to be robustly designed with frequent seal washing and automatic rinsing. Depending on the material used for the LC flow path, it might be appropriate to call this type of system “metal-free” or “stainless steel-free”. Technically speaking, it might be even more precise to call these systems “corrosion-resistant”.
In laboratories that do not have corrosion-resistant systems, it has become common practice to perform conditioning procedures that are meant to reduce the amount of metals eluting from a system. However, these procedures can be tedious and time‑consuming because they involve flushing the entire system with a chelator or oxidizing acid, for example, EDTA, citric acid, acetylacetone, 30% phosphoric acid, using an overnight process. This is a serious drawback when it comes to the laboratory and experimental productivity.
What Kinds of Materials Can be Considered as Being Bioinert and/or Biocompatible?
Most commonly, polyether ether ketone (PEEK), MP35N (a nickel‑cobalt alloy), and titanium are used as bioinert and biocompatible materials, but some systems also include ceramic, sapphire, gold-plated, high‑molecular-weight polyethylene, or fluoropolymer parts. These materials may not be low adsorption for all intended applications, however.
PEEK is probably the most frequently used material. PEEK can minimize protein adsorption, but there are some limitations. Since PEEK is an organic polymer, PEEK tubes are prone to swelling and pressure limitations (low mechanical stability). Alone as column hardware, PEEK can indeed be problematic as a result of variable chromatographic performance and short column lifetimes. Moreover, as a result of variability in PEEK manufacturing procedures, it is challenging to produce columns and tubing with consistent inner diameter. Sometimes PEEK is used as a liner within a metal-clad column hardware and tubing. These PEEK-lined components provide metal-free fluidic paths while maintaining the mechanical stability of the metal hardware. When applying PEEK as column frit material, low frit permeability is often observed due to the large dimensional (inner diameter) variability. In addition, PEEK is incompatible with some solvents. PEEK is also relatively hydrophobic and may require conditioning to avoid losses of hydrophobic analytes.
Alternative metals to stainless steel, such as titanium and MP35N, have been used for some applications because of their improved corrosion resistance. Exposure to air and water creates an oxide film at the surface of titanium materials, which generally ensures inertness (4). In addition, titanium is a good material for column and instrument manufacturing because it features mechanical properties close to those of stainless steel (5). Titanium components were first included in LC systems, but today titanium can also be found in column hardware, as a liner and as a frit material. Benefits from titanium column hardware have been reported (6), however, titanium exhibits some reactivity and some particular sample loss problems, for example, phosphorylated compounds, such that there is still work to be done on when and where it is appropriate to employ titanium parts. There are literature reports that passive titanium oxide was found to corrode upon exposure to anhydrous acetonitrile–methanol, releasing titanium ions that may adsorb on the stationary phase and cause peak tailing (4).
MP35N alloys are known for their ultrahigh strength, toughness, ductility, and outstanding corrosion resistance. Therefore, they are frequently used for valves, tubes, and needles in LC systems. They have also been shown to help minimize sample adsorption, even though there is still room to improve adsorption properties.
It has been reported that the inlet and outlet frits in a column are considered to be more critical for sample adsorption versus the wetted wall of the column tube (7,8). Consequently, vendors have developed titanium and PEEK frits. However, as mentioned earlier, both materials can potentially introduce their own type of adsorptive losses.
Ahybrid inorganic-organic surface technologywas also introduced that is based on ethylene-bridged siloxane chemistry formed on metal substrates. This hybrid surface has been demonstrated to mitigate interactions between samples and the metal surfaces of LC systems or columns (9), This hybrid surface is more hydrophilic than PEEK, making it less prone to hydrophobic adsorption. However, there might be methods in which these properties, albeit mitigated, are still a complication.
What are Problematic Samples and What Difference is Expected Between Conventional and Newer Corrosion-Resistant/Low Adsorption Systems?
Sample compounds containing phosphate or carboxylate groups, for example, nucleoside triphosphates, and solutes with other electron-rich functional groups have been reported to show low recovery and/or peak tailing when analyzed on LC systems equipped with stainless steel materials. For systems containing hydrophobic polymers, such as PEEK or PTFE components, large solutes with hydrophobic moieties, for example, peptides, proteins, protein aggregates, may show recovery issues. This can be attributed to undesired adsorptive interactions. These adsorptive interactions, or so-called secondary interactions, increase according to the number of functional groups within and the size of the analyte molecule.
Indeed, the adsorption of some analytes on stainless steel hardware can be serious. Titanium column hardware has been shown to provide higher aggregate (monoclonal antibody [mAb]) recovery in size-exclusion chromatography (SEC) (6). Titanium hardware and components have also been shown to improve the recovery of some test proteins during cation-exchange chromatography.
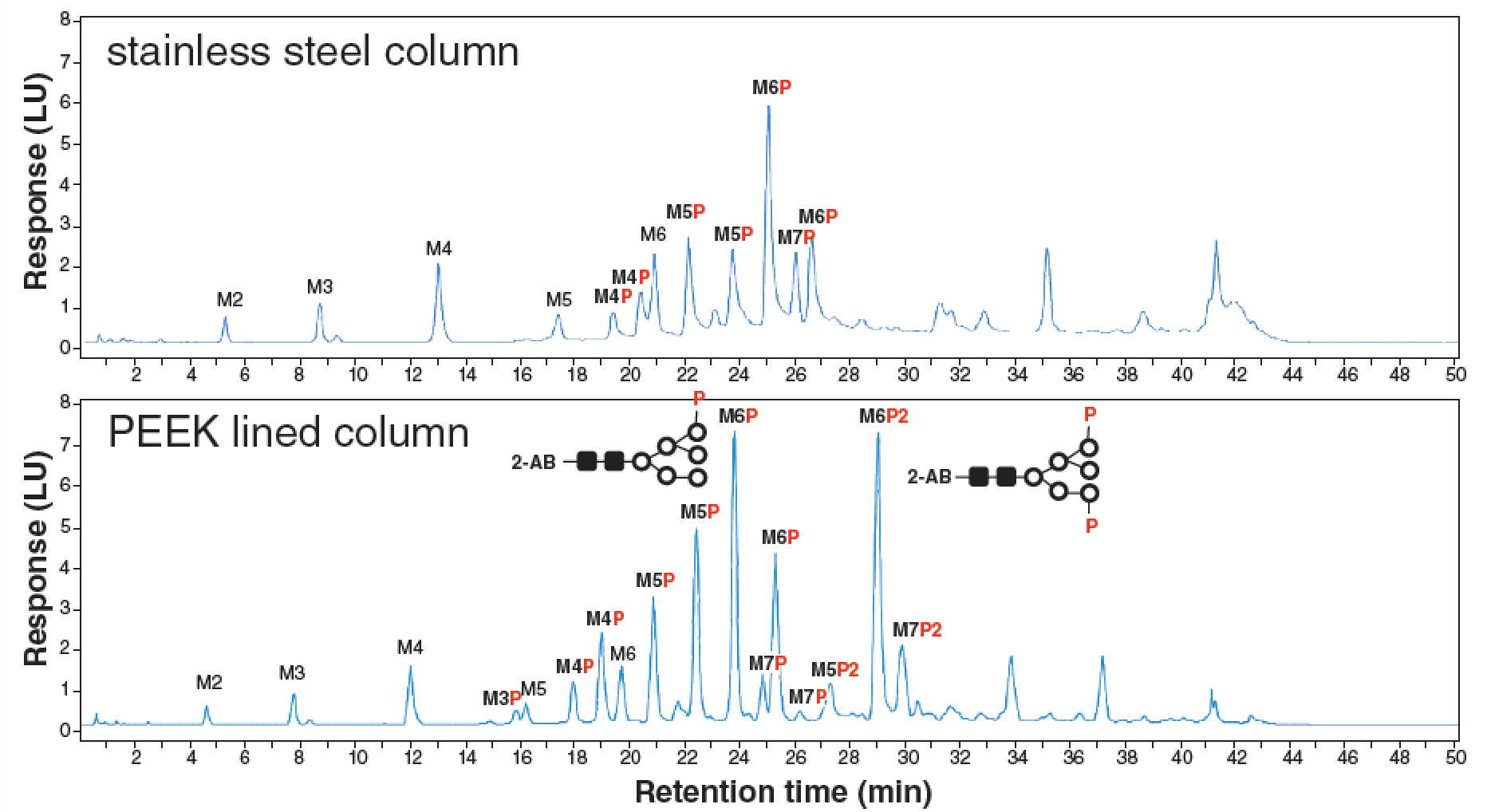
PEEK-lined reversed-phase columns have been reported to give superior recovery and sharper peaks compared to conventional hardware for nucleotides (ATP, ADP), for quinolinol and hinokitiol, and for intact and subunit monoclonal antibodies, as well as for some peptides. Hydrophilic interaction liquid chromatography (HILIC) is the gold standard for the analysis of N-glycans enzymatically released from biopharmaceuticals; however, poor recovery is often observed when analyzing phosphorylated N-glycans. It has been shown that those samples require a metal‑free flow path and low adsorption column hardware to avoid undesired interactions between the phosphate groups and stainless steel components (10). Figure 1 shows an example of labelled released glycan analysis in HILIC, performed using stainless steel and PEEK-lined columns.
The hybrid surface hardware has been shown to provide improved recovery and peak shape in reversed phase and hydrophilic interaction chromatographic modes for various compounds (9,11).
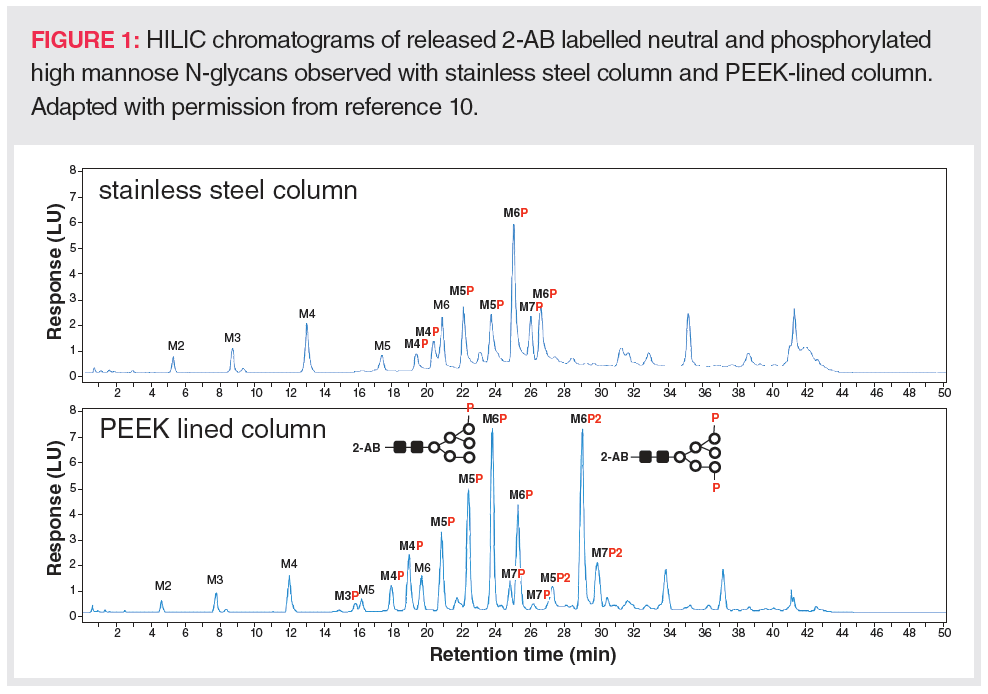
In addition to recovery issues, on-column methionine oxidation often takes place during LC tandem mass spectrometry (MS/MS) peptide mapping analysis of antibody‑based biotherapeutics when using stainless steel columns over the long term (12). Figure 2 shows an example of the artificial increase of methionine oxidation on an extensively used stainless steel column.
Conclusion
This article aimed to show the importance of asking questions and asking for terms to be defined. There is a need to use more descriptive and precise terms. It is probably more correct to say “low adsorption” and “corrosion-resistant” systems rather than biocompatible, bioinert, metal-free, or inert.
New technologies are now available for historically challenging separations. The applications for liquid chromatography are many and the demand for biomolecular chromatographic separations has never been stronger., Advances in LC will continue and the outlook for even more precisely controlled separations looks very promising.
Acknowledgement
The author wishes to thank Koen Sandra (RIC), Matthew Lauber, and Brian Murphy (Waters) for fruitful discussions and comments.

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)