Could This Be Your MS System of the Future?
LCGC North America
With the advances in LC-MS in recent years, is there another practical option on the horizon for those who need the specificity of MS and can justify the narrow definition of a purpose-built system?
In an astonishingly short time, mass spectrometer manufacturers have developed highly capable, compact instruments that integrate well with advanced liquid chromatography (LC) systems. Driving that achievement are breakthroughs in technology and manufacturing design, not to mention a universal desire to seek out and serve practitioners' needs. Consequently, the choice of instruments ranges widely. Some exotic hybrids offer ultrahigh resolution and confer novel benefits like ion mobility and a variety of interfaces. Others are low-resolution instruments that, despite their relatively low cost, nevertheless display capabilities akin to the best-performing instruments of a decade ago. Yet there might be another option on the horizon if the application is understood well enough and can justify the narrow definition of a non-LC or, perhaps, limited-LC introduction system.

Michael P. Balogh
In previous columns, we made glancing references to what we might lump together as nonanalytical uses of mass spectometry (MS) in airport security applications and for process instruments. In some cases, because of its greatly reduced vacuum requirements, a gas chromatography (GC)-only design edges analytical chemistry closer to a design that is less costly but still capable in the MS model. Relatively recently, two companies have emerged to raise interest in small, portable mass spectrometers. Simultaneously, some complementary technologies, such as thermal desorption, have excited interest because full sample preparation and LC separation are not required routinely.
So Why Isn't Every Bench Home to a Mini-MS System?
A frequent impediment to developing a new instrument of any kind is cost and expected market return. Startups may find it easier, at least initially, to introduce novel technologies than larger corporations because they often are burdened with less diverse financial demands and often focus resource in a singular product definition. The longer view of market potential eventually determines whether the technology becomes a commercially viable product, as opposed to a curiosity destined to become an historical footnote. For instance, the less selective instruments requiring high acquisition threshold settings employed today in airport security could be replaced by more selective mass spectrometers. Yet, for a market viewed as finite (estimates are of fewer than about 10,000 units, total), the level of support required, including training and upkeep, mitigates against development.
Nevertheless, two smaller companies, Torion Technologies, Inc. (American Fork, Utah) and Griffin Analytical (a business unit of ICx Technologies, West Lafayette, Indiana), are pursuing a broader vision. Both support small ion-trap technology using different basic approaches that fit applications such as illegal drug detection and other homeland security uses. A small suitcase-sized unit from Torion, the GUARDIAN 7, has illustrated its GC capabilities analyzing security samples and toxic industrial chemicals (1). The GUARDIAN 7 can sample directly from a solid-phase microextraction (SPME) membrane. Similarly, the Griffin design can acquire data from a sorbent-captured sample which is then brought to the MS. The 600 model from Griffin — while not exactly miniature — runs completely unattended.
Meanwhile, some researchers have forged ahead, relying on the recent and easily accessible return of thermal desorption called "ASAP" (atmospheric solids analysis probe). ASAP is a product of M&M Consulting (Hockessin, Delaware), which has commercialized it to fit a wide range of mass spectrometers. A probe design manufactured by Waters Corporation (Milford, Massachusetts) incorporates the ASAP technology suited for the entire current line of MS instruments. When this probe first appeared on the market, its potential and fundamentals were discussed in peer-reviewed journals and in this column (2–4). Apart from its commercially engineered advantages, the technology harkens back to early work by Horning and others. Horning exposed a liquid or solid sample placed upon an inert, solid probe to hot gas in the presence of ions. Thus, he created ions of interest (5). An oft-used diagram (Figure 1) indicates why a simple process that involves atmospheric pressure chemical ionization (APCI) (and perhaps atmospheric photoionization [APPI]) and the readily available nitrogen stream within an instrument's source works surprisingly well. Many samples from a variety of liquid and solid matrixes once volatilized are amenable to ionization by APCI and APPI without first diluting or otherwise preparing the sample.
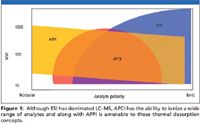
Figure 1
APCI overlaps much of what we consider the domain of electrospray ionization (ESI). ESI has been central to our practice for many years. Our thinking tends to be so "LC-centric," because we analyze polar compounds with it (as well as those that ionize readily from a reversed-phase solution) after separating or at least dissolving samples in solvent. So what many discover when they first use the ASAP probe is its sensitivity. Because we are conditioned to LC introduction, which requires us to dilute samples, users might be shocked at its apparent sensitivity. The sample is used directly, often in its final finished form, undiluted, and so at much higher concentration.
Advantages of Ion Traps in Miniature
Two articles published in 2004 and 2006 summed up the advantages and disadvantages of ion-trap technology (6,7). Because portable mass spectrometers require reduction in size, weight, and power consumption, important components like vacuum pumps must also undergo miniaturization. The ion-trap mass analyzer design is a good place to start. Inherently small, it operates with few ion-optic elements, which do not require a highly precise alignment (unlike other types of mass analyzers) to achieve their specifications. You can perform multiple stages of MS (fragmentation) in the same mass-analyzer space of these trapping devices. Moreover, the operating pressure for ion traps can be higher than that required for other forms of MS, making pumping requirements less stringent (6):
"(R)adio frequency . . . trapping voltage is inversely proportional to the square of the analyzer radial dimension, a modest decrease in analyzer size results in a large reduction in operating voltage and, hence, lower power requirements. An added potential benefit of the reduced analyzer size is the shorter ion path length, which may ease the vacuum requirements even further. This is an especially important area as some of the most limiting aspects of MS miniaturization are not in the ion optic components, but rather in the vacuum and other support assemblies."
The Lammert paper (6) addresses a downside of ion traps as the basis for the device:
"The ability to miniaturize ion trap mass spectrometers hinges on addressing the issues of space charge (8) and machining tolerance limits. Miniature ion traps with conventional ion trap geometries (i.e., hyperbolic surfaces) have been previously explored (9). However, as devices become smaller, the machining tolerances play an increasingly significant role in trapping field defects."
The limitations of space charge, significant for ion traps, are handled in various ways in larger commercial instruments. When a certain well-characterized number of ions is exceeded in a given space, the measured results (mass calibration and linearity response) are no longer dependable.
The toroidal design of the Torion instruments offers some advantages in miniaturization illustrated here over attempts so far (Figure 2), especially with 2D designs developed to overcome space charge effects or increase ion capacity on commercial instruments.
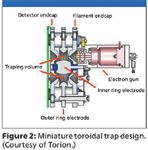
Figure 2
The Future of Mini-MS System Designs
Because both Griffin and Torion came into existence in 2001, sufficient time has elapsed to monitor how their technologies have performed, which until now have been predominantly GC-based analyses. Looking forward, Garth Patterson, chief technology officer at Griffin, takes a longer view. While technology and manufacturing issues are being resolved, the future lies in developing sample introduction (10) such as desorption electrospray ionization (DESI), direct analysis in real time (DART), and others.
Graham Cooks' group at Purdue (Purdue University, West Lafayette, Indiana), DESI was developed, gives us a view of how thermal desorption techniques might solve problems attendant with coupling them with miniaturized designs. A recent paper from the Cooks group sheds some light on how the limited stage design of a mini-MS might achieve improved sensitivity and linearity through discontinuous atmospheric ion introduction (DAPI) (11). The small, portable design referred to as the Mini-11 weighs a mere 5.0 kg (11 lb) with batteries. The device, which consumes less than 35 W, is only 12 cm wide and 22 cm long.
Thermal Desorption Update
I recently spoke with Gary Van Berkel, group leader and distinguished researcher of the Organic and Biological Mass Spectrometry Group at Oak Ridge National Laboratory (Oak Ridge, Tennessee). From the ORNL perspective, he characterized attempts made over many years to develop field-portable MS instruments for security as a holy grail-like quest. He said that people want research-MS capabilities at reasonable cost and, significantly, without consumables, which can dampen interest. Beyond what we might consider consumables in the laboratory, a required gas or liquid supply and dependence upon lasers or other high-level components renders the idea less attractive in the field.
Because of its low cost, ASAP fits the "no consumables" requirement more closely than some other thermal desorption techniques (Figure 3). Though it does require some form of heating (hot nitrogen suffices), the sole consumable is a melting-point capillary (perhaps even a reusable one, at that).
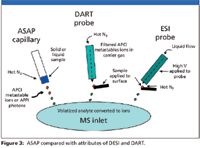
Figure 3
In a presentation at IMSC (Bremen, Germany) on August 30, 2009, Richard Fussell reported work underway at the Food and Environmental Research Agency (FERA, York, UK). FERA is investigating ways to test agriculture fields for contamination with chemicals like fungicides, which are used frequently in agriculture but which should not find their way into our food supply. Direct interrogation of the site as it stands with minimal sample handling is an attractive goal. Sample portability and protection of the sample's integrity "as sampled" before analysis must be assured and expectations of sensitivity and response must be met. The FERA group reported that 'field incurred' residues of strobilurin pesticides could be detected by simply stirring the ASAP probe directly into grains of wheat. Further research in the use of ASAP (or ambient ionization techniques if you prefer) is ongoing as part of the European Project on the use of new technologies for screening multiple contaminants in food. For more detail see the BioCop project (www.biocop.org).
Jackie Mosely, senior research officer in the chemistry department at Durham University (Dunham, UK) is also considering alternative means of employing the selectivity and specificity of MS. (Incidentally, Mosely's website is a good source of related information. Go to http://www.dur.ac.uk/chemistry/staff/, and click "Dr. Jackie A. Mosely.") In a recent conversation, she drew from her experience with Bruker Corporation (Billerica, Massachusetts) as well as her research and commented on three areas of interest. First, in research:
"The prospect of bringing a small MS into a specific, conditioned environment, such as a glove box, to handle compounds like pyrophoric chemicals, which are otherwise combustible in atmosphere, is attractive. Today our only choice is to build a glove box around the MS."
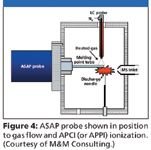
Figure 4
As her second case, Mosely points out the need for a simple sample introduction method:
"Synthetic chemists need only low resolution MS to quickly confirm their efforts. Without a utilitarian (mass spectrometer), equipped with something like an atmospheric insertion probe, they are required to do full-fledged sample prep, plus LC introduction for MS."
Finally, for her third case: "A small (mass spectrometer) would be a monumental advantage to teaching. The ability to introduce a sample easily to a small, portable (device) allows students a direct connection to being taught the fundamentals of chemistry."
A Quick Assessment Tool for the Synthetic Chemist
Peter J. Lee is a principal application chemist at Waters Corporation who holds an M.Eng. degree in organic synthesis from Kyushu University, Japan, and a Ph.D. in chemistry from Emory University (Atlanta, Georgia). With 50 articles and six U.S. patents, his research interests have included developing analytical methods used in food safety, environmental, pharmaceutical, and chemical analysis.
As an example of ASAP utility for synthetic chemists, Lee demonstrated a commonly used synthesis where a direct analysis might apply:
"In the chemical industry, the principal job of an organic chemist is to develop new chemicals and increase the production yield. In many cases, developing a new product or a new process with only a 5% increase in the yield can result in earnings of millions of dollars for (a) company. Organic chemists spend the majority of their time setting up reactions, monitoring the progress of reactions, working up reaction mixtures, and purifying products. They need to quickly obtain chemical structure data for making the right decisions about when to stop reactions and which fractions to pick to obtain products when monitoring reaction workup and purification processes."
The Sample Preparation Bottleneck
Time-consuming and labor-intensive sample workup procedures such as extraction, filtration, separation, and evaporation are typically required before analysis. Thin-layer chromatography (TLC) often is employed, which does not yield the specificity of MS (at least not without added steps). Chemists, whose expertise lies in analyzing new reactions and new compounds, in developing methods, and in data interpretation, must often wait hours for the data when using conventional tools such as nuclear magnetic resonance, LC–MS, or LC. Highly desirable technologies are those that can provide critical structure information quickly so that chemists can make informed decisions.
To illustrate the utility of ASAP as a direct means of interrogating a reaction without first preparing a sample, a reductive amination reaction based upon a well-referenced paper (12) was conducted. The reaction's progress was monitored using ASAP together with a small, tandem-quad mass spectrometer. Reductive amination is widely used in multiple-step organic syntheses for making agricultural chemicals, food additives, and building blocks for high performance materials (Figure 5). According to the paper, this reaction was typically run for 48 h, followed by approximately an hour-long reaction workup procedure. When analyzing this reaction mixture by LC or LC–MS methods preparative procedures are required to remove sodium cyanoborohydride (NaBH3CN), the reducing agent, which otherwise would plug ESI capillary tubes and ruin LC columns. Using ASAP, the reaction mixture was loaded directly onto the sealed-glass, melting-point capillary tube of the probe for MS analysis, without workup or sample cleanup.
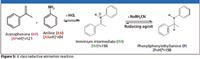
Figure 5
The ASAP probe was inserted into the sealed source enclosure, and the desolvation gas was heated rapidly to 400 °C. Data acquisition was 30 s, in positive mass scan mode. The source temperature was 120 °C, and the desolvation gas flow 500 L/h. Combined mass spectra were obtained by subtracting the baseline of the reference scans from the samples' total ion current profiles.
The reaction (Figure 6) was monitored by following the disappearance of the starting materials:
- The spectrum in Figure 6a was acquired after mixing acetophenone (AP) and aniline (AN) in methanol.
- The spectrum in Figure 6b was acquired 2 min after adding acid and shows the formation of an imminium intermediate (IM).
- The spectrum in Figure 6c, acquired 10 min after adding the reducing agent, shows the formation of the product, phenyl(phenylethyl)amine (P) and the disappearance of the imminium intermediate.
- The spectrum in Figure 6d, acquired 40 min after adding the reducing agent, shows the increase of the amine product (P) and the decrease of the starting reactants (AP and AN).
- The spectrum in Figure 6e was acquired 4 h after adding the reducing agent and reveals no remaining reactants, indicating the completion of the reaction.

Figure 6
Just a few years ago, the ability to do away with sample preparation was touted — perhaps overoptimistically — as hope for the future of atmospheric thermal desorption. As often happens when a technology comes of age and is applied to solving real problems, the end result is far better in combined use with other technologies. A small refinement to this process shows that sample prep is not always a chore best avoided. For example, an acetic anhydride reaction benefits from a quick preparative cleanup, thus dispensing with the burden of trying to characterize a complex MS spectrum that had not first undergone LC separation.
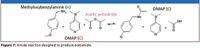
Figure 7
Amide formation is one of the most important reactions in organic synthesis. In this case (Figure 7), Lee set up a reaction by mixing methyloxybenzylamine (A) with base catalyst 4-dimethylaminopyridine (C) in CH3CN and adding acetic anhydride to produce acetamide. The spectrum in Figure 8b indicates the reaction was complete in 5 min. The spectra in Figures 8c and 8d show the product, acetamide, after a simple nonquantitative purification (PoraPak Rxn CX cartridge, Waters Corporation) to enhance the presence of ions of interest.

Figure 8
Future possibilities
We have already seen small, portable-MS devices combined with a variety of introduction techniques. The use of SPME by Torion and others; Griffin's adaptation of sorbent technology, which allows them to automatically desorb and downloads the sample information for real-time analysis; and Fussell's work taking site-specific samples to be analyzed are among the notables. Time-based and volume-based samples on reusable substrates that can be hand carried to the instrument or introduced to an instrument in the field are quite real.

Figure 9
On-site instruments such as the somewhat larger Griffin 450 (13), are more common but benefit from the overall advances in MS of recent years. Such instruments can be mounted and operated in manned, moving vehicles and from unmanned aerial or ground vehicles. Moreover, they lend themselves to on-site detection, quantification, identification, and confirmation of explosives and chemical warfare agents as well as toxic industrial chemicals.
A recently filed patent indicates continued interest in the broader application of thermal sampling and delivery techniques (14):
"(A)n open probe method for sample introduction into a mass spectrometer . . . comprising the steps of: loading a sample holder with sample compounds to be analyzed (into) a (heated) probe oven; flowing inert gas; vaporizing said sample by the combined effect of oven temperature and inert gas flow; entraining (the) vaporized sample in inert gas; and, transferring vaporized sample in inert gas into an ion source of a mass spectrometer."
Similar to some other current, thermal desorption techniques, the primary advantage claimed is that the "heated probe oven remains open to the ambient atmosphere during sample introduction and analysis, thereby enabling faster sample analysis."
The ability to combine techniques such as ASAP with instruments capable of highly selective, specific, MS determinations necessarily depends upon other advances. An essential aspect of any analysis is being able to reproduce and trust the data acquired. MS tools such as automated calibration and optimization are being offered increasingly by manufacturers to answer the increasing diversity of skill and applications for which MS is being sought.
In recent years, advances in software and hardware control permitting routine calibration and setup of mass spectrometers have convinced even skeptics that such a mass spectrometer's output can be trusted for a variety of analytical experimental needs. Because what we might call the "reintroduction of thermal desorption techniques," some six years ago, evidence supports some of the early assertions. Clearly, the techniques follow the fundamentals upon which they are based, yet with some added understanding. For example, DESI tends to ionize materials, much as ESI might does, and DART and ASAP materials behave much as APCI would. The lack of dilution in both DART and ASAP tends to effect a sometimes surprising sensitivity. Also the range of compounds ionized by APCI surprises many who have had little opportunity or need to employ it. Claims of no-sample-prep simplicity, while often true, have sometimes been overstated. In fact, intelligently employed sample preparation is often a key element of success with these techniques, and automation is still largely a work in progress. Otherwise, claims of a plug-and-play analytical capability might not be that far off.
Michael P. Balogh "MS — The Practical Art" Editor Michael P. Balogh is principal scientist, MS technology development, at Waters Corp. (Milford, Massachusetts); a former adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island); cofounder and current president of the Society for Small Molecule Science (CoSMoS) and a member of LCGC's editorial advisory board.
References
(1) D.W. Later, T.C. Wirth, C.R. Bowerbank, E.D. Lee, and C.S. Sadowski, Homeland Security, Supplement to LCGC and Spectroscopy, April 2009, p. 6.
(2) M.P. Balogh, LCGC 25(11), 1184–1195 (2007).
(3) M.P. Balogh, LCGC 25(3), 368–381 (2007).
(4) C.N. McEwen, R.G. McKay, and B.S. Larsen, Anal. Chem. 77, 7826–7831 (2005).
(5) E.C. Horning, M.G. Horning, D.I. Carroll, I. Dzidic, and R.N. Stillwell, Anal. Chem. 45, 936–994.
(6) S.A.Lammert, A.A. Rockwood, M. Wang, M.L. Lee, E.D. Lee, S.E. Tolley, J.R. Oliphant, J.L. Jones, and R.W. Waite, J. Am. Soc. Mass Spectrom. 17, 916–922 (2006).
(7) M.G. Blain, L.S. Riter, D. Cruz, D.E. Austin, G. Wu, W.R. Plass, and R.G. Cooks, Int. J. Mass Spectrom. 236, 91–104 (2004).
(8) S. Guan and A.G. Marshall, J. Am. Soc. Mass Spectrom. 5, 64–71 (1994).
(9) R.E. Kaiser, R.G. Cooks, G.C. Stafford, J.E.P. Syka, and P.H. Hemberger, Int. J. Mass Spectrom. 106, 79 (1991).
(10) L. Gao, A. Sugiarto, J.D. Harper, R.G. Cooks, and Z. Ouyang, Anal. Chem. 80, 7198–7205 (2008).
(11) L. Gao, G. Li, Z. Niea, J. Duncan, Z. Ouyang, and R.G. Cooks, Int. J. Mass Spectrom. 283, 30–34 (2009).
(12) R.F. Borch, M.D. Bernstein, and H.D. Durst, J. Am. Chem. Soc. 93, 2897 (1971).
(13) National Forensic Science Technology Center (NFSTC) Testing and Evaluation Report: Griffin 450 GC/MS, 11/28/2007 www.nfstc.org/lab/technology-evaluations.
(14) Amirav, Aviv, US patent application 20100019140, Open Probe Method and Device for Sample Introduction for Mass Spectrometry Analysis, January 28, 2010.
Erratum
In the February 2010 "MS—The Practical Art," the symbol for relative atomic mass appears as Ar. According to current use, it should be shown as Ar(E).
In addition, two of the equations on p. 128 should have read:
"1330/123 = 10.8"
"9131.8/100 = 91.3"

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






