Comparison of Fully and Superficially Porous Particle Columns for the Analysis of Basic Compounds
Since the introduction of high-performance liquid chromatography (HPLC) nearly 40 years ago, many improvements have been made to column stationary phases to achieve faster, more efficient separations. HPLC columns containing superficially porous (sometimes called fused-core) particles have recently gained increasing attention. Though this technology is not entirely new, it has been improved to the point where rapid, highly efficient separations can be achieved for some applications.
Since the introduction of high-performance liquid chromatography (HPLC) nearly 40 years ago, many improvements have been made to column stationary phases to achieve faster, more efficient separations. HPLC columns containing superficially porous (sometimes called fused-core) particles have recently gained increasing attention. Though this technology is not entirely new, it has been improved to the point where rapid, highly efficient separations can be achieved for some applications.
The goal of this work is to investigate the differences in separation performance between fully and superficially porous particle columns for routine analysis of basic pharmaceutical drug compounds. Major parameters of comparison are peak capacity (PC) and column efficiency at different mass loads.
Results
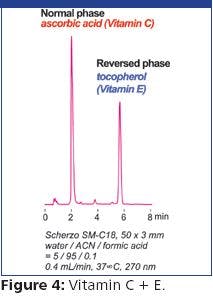
Figure 1 shows the comparison between XBridge, SunFire, and HALO columns for the separation of a standard mixture of basic compounds in a low pH mobile phase. The peak capacity for XBridge and HALO columns are nearly identical. The SunFire column has almost 60% higher peak capacity than the HALO column under the same conditions.

Figure 1
The best conditions for analysing basic compounds by HPLC are with high pH mobile phases. It is possible to operate XBridge columns at pH values up to 12 with exceptional lifetime. Figure 2 shows the difference in peak shape and signal intensity for amitriptyline analysed with low and high pH mobile phases on an XBridge C18 column. The efficiency for amitriptylene at high pH was more than 10× higher than at low pH.

Figure 2
Conclusions
The benefits of using appropriate fully porous particle HPLC columns with comparable particle size clearly outweigh those of using current superficially porous (fused-core) particle columns for the analysis of basic compounds. At high pH, XBridge columns demonstrated a 10-fold increase in column efficiency over low pH at the same mass loads. This dramatic advantage of hybrid particle technology is most important for increasing throughput in preparative HPLC separations, as well as increasing capacity for stability indicating methods of APIs.
For the complete application note, visit www.waters.com/28251
© 2009 Waters Corporation. Waters, The Science of What's Possible, SunFire and XBridge are trademarks of Waters Corporation. HALO is a trademark of Agilent Technologies Inc.

Waters Corporation
34 Maple Street, Milford, MA 01757
tel. (508)478-2000, fax (508)478-1990
Website: www.waters.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)