Comparing the Capabilities of Time-of-Flight and Quadrupole Mass Spectrometers
Special Issues
The authors explain some of the primary differences between quadrupole and time-of-flight mass analyzers and provide information regarding the benefits of each in their use for gas chromatography applications.
The quadrupole mass spectrometer is a scanning mass analyzer that uses the stability of ion trajectories in oscillating electric fields to separate them according to their mass-to-charge ratios (m/z). It comprises four parallel rods arranged as shown in Figure 1. A constant DC potential is applied to each rod pair, one pair positive and the other negative, on opposite planes. An alternating potential with frequency in the region of radio waves is superimposed over the DC potential on each rod pair. This radio frequency (RF) voltage causes the ions to spiral as they traverse the quadrupole toward the detector. This arrangement acts as a high pass m/z filter in one plane (+), and a low pass m/z filter in the other plane (–). Typically, the DC and RF potentials are adjusted so that only one m/z at a time can traverse the quadrupole. All others collide with the rods and are neutralized. To acquire a mass spectrum, the DC and RF potentials must be scanned in constant ratio across a range. Therefore, acquiring a broader mass range requires a longer scan time.
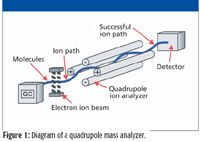
Figure 1
There are pros and cons to this type of analyzer. The advantages of a quadrupole system include its low cost, ability to perform both qualitative and quantitative analyses, and increased sensitivity capability through selected ion monitoring (SIM) mode. The major cons of quadrupole analyzers are that their scanning nature limits the acquisition rate and leads to spectral bias. The consequences of each will be discussed in more detail later.
TOF-MS
A time-of-flight (TOF) mass spectrometer is a nonscanning mass analyzer that emits pulses of ions (or transients) from the source. The ions are accelerated so that they have equal kinetic energy before entering a field free drift region, also known as the flight tube. See Figure 2 for a diagram of a TOF-mass spectrometry (MS) system. Because kinetic energy is equal to ½ mv2, where m is the mass of the ion and v is the ion velocity, the lower the ion's mass, the greater the velocity and shorter its flight time. The travel time from the ion source through the flight tube to the detector can be transformed to the m/z value through the relationships described previously. Because all ion masses are measured for each transient, TOF mass spectrometers are well-suited for the analysis of both targeted and nontargeted analytes. A nonscanning instrument such as a TOF-MS system offers many advantages including fast acquisition rates, spectral continuity, and exceptional dynamic range. The ability to acquire full mass range spectra without sacrificing speed or sensitivity makes TOF-MS an excellent choice for qualitative and quantitative analyses across a wide dynamic range in the presence of complex matrices.
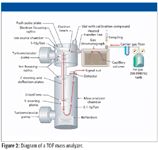
Figure 2
The following capabilities of TOF and quadrupole mass analyzers will be compared in more detail throughout this article: acquisition rates, spectral continuity, deconvolution capability, and quantitative abilities.
Acquisition Rates
Acquisition rate is a very important factor to consider when choosing an MS detector for gas chromatography (GC). With advances in the technology of GC, including narrow-bore capillary GC, high-speed GC, and comprehensive two-dimensional GC (GC×GC), peak widths are becoming narrower. This is creating a need for an MS detector with acquisition rates capable of obtaining enough data points to sufficiently define them. Table I shows an example of chromatographic peak widths and the acquisition rates recommended for successful quantitative analyses.
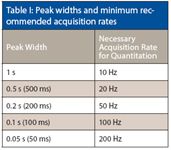
Table I: Peak widths and minimum recommended acquisition rates
For quantitative analyses, it is widely accepted that a detector capable of delivering ≥10 data points across a baseline resolved chromatographic peak is necessary. Peak widths less than 500 ms will not be defined sufficiently using a quadrupole MS system operating at a 20-Hz acquisition rate. This makes their use for high speed GC and GC×GC insufficient as these applications can generate peaks narrower than 50 ms.
It should be noted that a quadrupole MS system allows maximum scanning rates of approximately 10,000 u/s. This means that the wider the scan range acquired, the slower the acquisition rate and vice versa. See Table II for typical mass ranges and their associated maximum scan rates.

Table II: Quadrupole mass ranges and associated maximum scan rates
A TOF-MS system, however, is capable of acquisition rates up to 500 spectra/s independent of mass range, making it an excellent choice as an MS detector for high-speed GC and GC×GC applications.
Mass Spectral Deconvolution
Both quadrupole and TOF-MS systems are capable of utilizing the power of mass spectral deconvolution algorithms. There are, however, two major advantages to TOF-generated mass spectra with regard to the performance of deconvolution algorithms.
First, the higher acquisition rate capability of TOF provides the data density necessary for successful mass spectral deconvolution. Results have been published showing that 18–20 data points across a peak are necessary for accurate quantitative deconvolution of chromatographically unresolved peaks (1). Figure 3 shows the effect of acquisition rate on deconvolution performance. This example shows a region of a GC–TOF-MS chromatogram in which nine chromatographically unresolved pesticides are eluted. In the top example, an acquisition rate of 40 spectra/s was used and all nine analytes were located and identified. In the middle example, an acquisition rate of 10 spectra/s was used and six analytes were located and identified. In the bottom example, an acquisition rate of 2 spectra/s was used and none of the analytes were located.

Figure 3
Spectral Continuity
The second major (and perhaps the most important) difference between TOF and scanning instruments is TOF's spectral continuity. This is extremely important for mass spectral deconvolution. With one particular system, 5000 pulsed transients (full mass range spectra) are analyzed per second (Pegasus HT TOF MS, LECO, St. Joseph, Michigan). During this time, ion packets are formed, extracted, and mass analyzed almost simultaneously. Therefore, concentration changes occurring in the source during peak elution do not affect spectral continuity in TOF-MS. The result is nonskewed mass spectra that allow optimal performance of the mass spectral deconvolution algorithms.
With quadrupole MS or other scanning instruments, a scan is initiated at one point in time. As the scan progresses, time elapses and therefore, the last data for the scan is collected at a second point in time. During the scanning process, analyte concentrations change in the source, resulting in skewed spectra. The skew is corrected by averaging spectra across the apex of the chromatographic peak. This significantly complicates the mathematics of deconvolution in the case of overlapped spectra. Figure 4 shows an example of the spectral continuity (left spectra) offered by TOF-MS as compared to the skewed spectra (right spectra) typical of scanning instruments.

Figure 4
Figure 5 shows an example of a juniper oil sample that was analyzed under identical chromatographic conditions on both quadrupole and TOF-MS detectors. As a result of factors discussed in this document, such as acquisition rate and spectral continuity, the advantages of TOF-MS are made evident. In this example, 137 components were detected and identified by TOF-MS with an average library match of 85%. In the same sample, only 96 components were detected and identified on the quadrupole MS with an average library match of 75%.

Figure 5
Quantitation Capabilities
There appear to be common misconceptions about TOF with regard to its abilities as a quantitative analytical tool. These misconceptions might stem from the lack of success of earlier TOF instruments. Many people also confuse matrix-assisted laser desorption ionization (MALDI)-TOF with the TOF systems associated with GC applications, which include electron ionization (EI)-TOF and chemical ionization (CI)-TOF.
TOF-MS instruments are quite capable of performing successful quantitative analyses. In fact, they offer some advantages over quadrupole instruments for quantitation. These advantages include increased dynamic range and the ability to perform reproducible quantitative analyses effectively without the need to run in SIM, typical for quadrupole instruments. Figure 6 shows a head-to-head comparison of the linear dynamic ranges for a quadrupole system and a TOF system. In this figure, the curves represent detector response versus concentration of hexachlorobenzene (HCB). Each GC–MS instrument was configured with identical conditions. HCB standards were analyzed over a concentration range from 2 to 20,000 pg on column. These curves demonstrate that the dynamic range on the quadrupole covers three orders of magnitude (2–2000 pg), while the TOF covers four orders (2–20,000 pg).

Figure 6
Table III shows a comparison of percent relative standard deviation (RSD) values for 10 replicate injections of a standard containing octafluoronaphthalene (OFN), dibromobenzene (DBB), tribromobenzene (TBB), benzophenone (BZP), tetrabromothiophene (TBTP), hexachlorobenzene (HCB), and hexabromobenzene (HBB). The values represent the RSD for peak areas based upon a single quantitation mass for each analyte. The results from 10 replicate injections performed on a quadrupole operating in scan and SIM modes were compared to those generated on a TOF system, which is always collecting full-range mass spectra. These results show that a quadrupole instrument's reproducibility suffers when operating in scan mode rather than SIM. This is one of the reasons SIM is most often chosen for quantitative analyses. Unfortunately, this necessitates samples with nontargeted compounds to be analyzed twice: once in scan mode to identify compounds present and again in SIM mode for best quantitative results. TOF, on the other hand, can provide repeatable quantitative results and full range mass spectra for qualitative identification in a single analysis. This streamlines the quantitation process for samples containing nontargeted compounds.
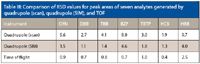
Table III: Comparison of RSD values for peak areas of seven analytes generated by quadrupole (scan), quadrupole (SIM), and TOF
Conclusion
Both quadrupole and TOF-MS systems are excellent choices as detectors for gas chromatography. The key is to understand which is best suited for your particular application.
Quadrupole systems offer an affordable solution for routine target compound analyses in which matrix interference is not abundant and also allow for enhanced sensitivity by operating in the SIM mode.
TOF-MS is the detector of choice for fast GC separations, GC×GC applications, and for the analysis of target and nontarget compounds in the presence of complex matrices wherein many chromatographically unresolved peaks are likely.
Joe Binkley and Mark Libardoni are with LECO, St. Joseph, Michigan.
References
(1) ChromaTOF Auto Peak Find and Deconvolution: http://www.leco.com/resources/application_note_subs/pdf/separation_science/performance_notes/209-076-016.pdf

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













