Chromatography and COVID-19
Szabolcs Fekete, winner of the HTC 2022 Innovation Award, reveals the benefits of ultra-short columns to investigate COVID-19.
Q. Your work on the innovative potential of ultra-short columns has been praised highly by key opinion leaders in the chromatography community. Briefly, what was innovative about the development of ultra-short columns and how did this challenge conventional wisdom?
A: It was already known, and has been studied in the past that, as a result of the on/off elution mechanism observed with large solutes, such as proteins and nucleic acids, in liquid chromatography (LC), the column length has less impact on controlling their retention compared to small solutes (1,2). More precisely, under commonly used linear gradient conditions, only a short inlet segment of a column (0.1–1 cm) effectively retains the large molecules (3). One might refer to this “useful” inlet column section as the “effective column length”. For example, when running a fast linear gradient for an intact monoclonal antibody (mAb) in reversed‑phase LC, an effective length of shorter than 0.5 cm is expected. After the solute passes this inlet section, its local retention factor will be very low, allowing for an even shorter column length with faster gradients.
Therefore, we challenged the current practice of leveraging 10–25-cm long columns for reversed-phase LC with a novel 0.2–2-cm long column format. As we predicted, equivalent separations could be performed on these ultra‑short columns, allowing for a 0.5–1 min analysis time instead of the routine 10–30 min. The main challenge was to redesign the column hardware to minimize the “extra-bed” volume within the column and to estimate the effect of system band dispersion when using these very small volume columns on commercial ultrahigh-pressure liquid chromatography (UHPLC) systems. We found that 15 × 2.1 mm and 20 × 2.1 mm dimensions provided the best compromise between analysis time and efficiency loss caused by extra-bed and extracolumn dispersion (4). The redesign has allowed for very efficient gradient separations of mAbs and antibody-drug conjugate (ADC) samples in the 0.5–1 min interval.
The ultra‑short columns can be used with other retentive chromatographic modes, including ion-exchange chromatography (IEC), hydrophilic interaction liquid chromatography (HILIC), and hydrophobic interaction chromatography (HIC) (5,6).
Q. You recently used these columns to speed up the analysis of COVID-19 antibody therapeutics (6). What was the rationale behind this research?
A: The rationale behind this research was the COVID-19 pandemic, which necessitated the emergency use authorization (EUA) of several new therapeutics and vaccines. With an EUA submission, characterization data on a new drug candidate and drug development are often performed simultaneously. Hence, there is little time for misguided method development or traditional turnaround times. We have taken this situation as a chance to propose new means of speeding up the chromatographic characterization of protein therapeutics. To achieve high‑throughput reversed‑phase LC, IEC, HILIC, and size-exclusion chromatography (SEC) separations for two COVID-19‑related mAbs (casirivimab and imdevimab), automated systematic retention modelling was combined with the ultra-short columns (6). The optimized chromatographic methods allowed for the comprehensive characterization of a COVID-19 mAb cocktail with a sub-1-h turnaround time. Retention models were also developed, facilitating the virtual transfer of methods without the need for wet experiments, reducing the time needed for development and optimization. This approach presents laboratories with an easily deployable method supported on ultra-short and conventional column formats.
Q. Can you describe the individual applications you investigated and their benefits for the analyst?
A: We have developed a variety of applications on ultra-short columns, with one of the most interesting being the demonstration of a short bed column (15 × 2.1 mm) packed with an optimized stationary phase for LC–high-resolution mass spectrometry (HRMS) quantitation of intact proteins (7). This new column allowed rapid separations, facilitated attomole levels of detection, and could be successfully applied to detect and characterize mAbs in biofluids when paired with a new aptamer-based immunocapture approach. Another study reported the use of an ultra-short column for very high temperature, mAb-complex separations (8). In reversed-phase LC, mAb complexes may require extremely harsh, superheated conditions to dissociate and elute as interpretable profiles. On-column degradation is a consequence of superheated conditions, and the application of ultra-short columns and fast gradients serve as a potential solution to reduce degradation by-products and analysis time.
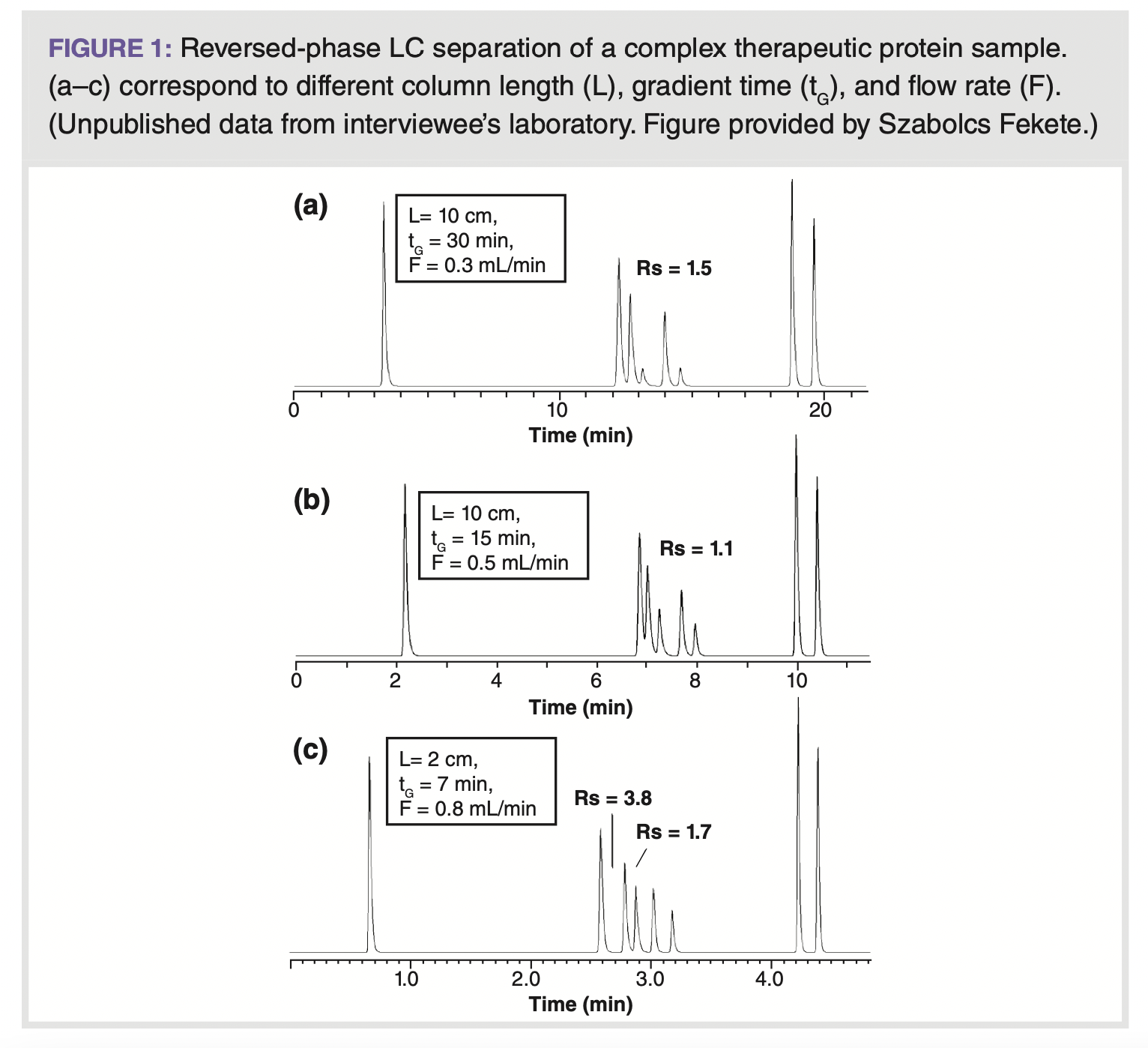
We also recently applied a very unusual approach to decrease the intrinsic gradient steepness of a reversed-phase LC method to separate a complex therapeutic protein sample in 6–8 min. Based on scouting experiments, we found that a small gradient slope (flat gradient) favoured separation. Normally flat gradients are performed by either increasing the gradient time and/or flow rate, or by decreasing the absolute change of mobile phase composition (narrower ΔB% range), leading to a longer analysis time. Instead, we had the idea to decrease the intrinsic gradient steepness using an ultra-short column, which has a low column volume (please note that the intrinsic gradient steepness is proportional with the column volume; however, this fact is not often considered). Figure 1 illustrates the impact of gradient steepness on the separation of this complex protein sample. The chromatograms in Figure 1(a) and 1(b) used a 10-cm long column, while Figure 1(c) corresponds to a 2-cm long column. Using ultra‑short columns, the analysis time was kept at 7 min—countering the commonly accepted notion that longer columns are required for better resolution.
Q. What were the main analytical challenges you had to overcome?
A: We were extremely confident about the analytical workflow developed for protein separations using ultra-short columns, multi-isocratic elution mode, and on-column fractioning, and were convinced that any sample could be separated. However, we indeed faced an issue with one project that resulted in a set of closely eluting intact proteins. We attempted a number of conditions, which included one of our PhD students evaluating various multi‑isocratic programmes. One day she programmed a negative step gradient that finally solved the issue and a baseline separation could be attained, leading to subsequent work for us to understand how large molecules elute under these conditions (9). To be honest,
I have never seen the use of multiple isocratic steps together with the addition of a steep negative gradient segment. When inserting a negative segment by decreasing the eluent strength, a slightly more retained peak can be parked somewhere along the column while a slightly less retained compound
is eluting. This negative segmented multi-isocratic elution mode can potentially be applied to improve the separation of notoriously heterogeneous biopharmaceutical samples to separate critical peak pairs (for example, intact mAb variants, Fc-fusion proteins, bispecific‑mAb, antibody mixtures, or ADC species). Moreover, an equidistant peak distribution can be achieved if desired.
Another developing challenge is the nonspecific adsorption and undesired secondary interactions between column hardware and biomolecules that continue to plague laboratories, presenting numerous analytical challenges. Novel hydrophilically modified hybrid surfaces continue to expand and facilitate the deployment of more robust and sensitive analyses (10). This approach advances separations with aqueous mobile phases in both SEC and ion exchange for proteins and nucleic acids.
Q. What else are you working on?
A: Recently, we illustrated the potential of so-called “dual stationary phase gradients” (11), corroborating previous reports that a stationary phase gradient can produce selectivity in a separation as effectively as a mobile phase gradient programme—especially under mobile phase gradient elution conditions. In reality, no column is based on one type of interaction mechanism, with many designed to elicit multiple solute to stationary phase interactions. These mixed‑mode columns inspired us to give more careful consideration to the idea of dual stationary phase gradients. With the theory applied in our recent work, modulation of two unique interaction mechanisms across a chromatographic column has the potential to provide previously unseen selectivity. With the increasing prevalence of mixed‑mode columns, we believe there will be ample opportunity to explore these new concepts in experimental work.
We have also presented at HPLC 2022 and ISC 2022 on so‑called “pressure‑enhanced liquid chromatography” (PE-LC), which leverages pressure changes to alter selectivity (12). An apparatus for the facile manipulation of column pressure was assembled through a two-pump system and postcolumn flow restriction, allowing us to program various constant pressure changes and even pressure gradients. With our proposed setup, it was possible to combine eluent and pressure gradients in the same analytical run, improving the separation of insulin from one of its forced-degradation impurities. We believe that this novel PE‑LC approach opens up new possibilities in liquid chromatography, especially for samples containing solutes of moderate to large sizes. I continue to work on understanding peak formation under these pressure gradients and how PE-LC may benefit from large peak compression effects under negative pressure gradients.
We also have numerous ideas and research projects primarily on the chromatographic separation of new modalities such as cell and gene therapies, focusing on new size- and charged-based separations and trying to understand their underlying phenomena.
I feel extremely lucky for the continued support of my colleagues during this work and want to thank Davy Guillarme, Jean-Luc Veuthey, and Amarande Murisier (University of Geneva), and Matthew Lauber, Jennifer Nguyen, and Mike Fogwill (Waters Corporation) for all of their help and contributions.
References
- Snyder, L. R.; Stadalius, M. A.; Quarry, M. A. Gradient Elution in Reversed-Phase HPLC. Anal. Chem. 1983, 55, 1412A−1430A. DOI: 10.1021/ac00264a716
- Stadalius, M. A.; Quarry, M. A.; Snyder, L. R. Optimization Model for the Gradient Elution Separation of Peptide Mixtures by Reversed-Phase High-Performance Liquid Chromatography: Application to Method Development and the Choice of Column Configuration. J. Chromatogr. A 1985, 327, 93−113. DOI: 10.1016/S0021-9673(01)81640-X
- Fekete, S.; Bobály, B.; Nguyen, J. M.; et al. Use of Ultrashort Columns for Therapeutic Protein Separations. Part 1: Theoretical Considerations and Proof of Concept. Anal. Chem. 2021, 93, 1277–1284. DOI: 10.1021/acs.analchem.0c04082
- Fekete, S.; Murisier, A.; Nguyen, J. M.; et al. Use of Ultra-short Columns for Therapeutic Protein Separations, Part 2: Designing the Optimal Column Dimension for Reversed-Phase Liquid Chromatography. Anal. Chem. 2021, 93, 1285–1293. DOI: 10.1021/acs.analchem.0c01720
- Navarro-Huerta, J. A.; Murisier, A.; Nguyen, J. M.; et al. Ultra-Short Ion-Exchange Columns for Fast Charge Variants Analysis of Therapeutic Proteins. J. Chromatogr. A 2021, 1657, 462568. DOI: 10.1016/j.chroma.2021.462568
- Duivelshof, B.; Zoldhegyi, A.; Guillarme, D.; Lauber, M.; Fekete, S. Expediting the Chromatographic Analysis of COVID-19 Antibody Therapeutics with Ultra-Short Columns, Retention Modeling and Automated Method Development. J. Pharm. Biomed. Anal. 2022, 221, 115039. DOI: 10.1016/j.jpba.2022.115039
- Nguyen, J. M.; Liu, X.; DeLoffi, M.; et al. Aptamer-Based Immunoaffinity LC–MS Using an Ultra-Short Column for Rapid Attomole Level Quantitation of Intact mAbs. J. Chrom. B 2021, 1173, 122694. DOI: 10.1016/j.jchromb.2021.122694
- Bobály, B.; Keresztfalvi, A.; Graber, T.; Schwarz, M. A. Superheated Reversed Phase Chromatography with Ultrashort Columns for the Analysis of Therapeutic Proteins. J. Pharm. Biomed. Anal. 2021, 203, 114462. DOI: 10.1016/j.jpba.2021.114162
- Fekete, S.; Murisier, A.; Nguyen, J. M.; Lauber, M. A.; Guillarme, D. Negative Gradient Slope Methods to Improve the Separation of Closely Eluting Proteins. J. Chromatogr. A 2021, 1635, 461743. DOI: 10.1016/j.chroma.2020.461743
- Fekete, S.; DeLano, M.; Harrison, A. B.; et al. Size Exclusion and Ion Exchange Chromatographic Hardware Modified with a Hydrophilic Hybrid Surface. Anal. Chem. 2022, 94, 3360–3367. DOI: 10.1021/acs.analchem.1c05466
- Fekete, S.; Lauber, M. A. Studying the Possibilities of Dual Stationary Phase Gradients to Explore Alternative Selectivities in Liquid Chromatography. J. Chromatogr. A 2022, 1681, 463492. DOI: 10.1016/j.chroma.2022.463492
- Fekete, S.; Fogwill, M.; Lauber, M. A. Pressure-Enhanced Liquid Chromatography, A Proof of Concept: Tuning Selectivity with Pressure Changes and Gradients. Anal. Chem. 2022, 94, 7877–7884. DOI: 10.1021/acs.analchem.2c00464
Szabolcs Fekete worked in the pharmaceutical industry in analytical R&D for 10 years before moving to the University of Geneva in Switzerland and working as a postdoc and later as scientific collaborator for a decade. In April 2021, he joined Waters Corporation and now works as a consulting scientist. He has contributed to ~170 peer-reviewed journal articles, co-authored 10+ book chapters, and co-edited three handbooks. His current interests include: separations of new chemical modalities (including oligonucleotides, RNA therapeutics, proteins, antibody-drug conjugates, and gene therapy products), fundamentals of chromatography, column technology, new method development approaches, and modelling. He is well known for his various collaborations with both academic and industrial partners.
He has an h-index of 47, with over 6700 citations (2023, January, Google Scholar).

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









