Characterization of Recombinant Protein Biotherapeutic Using TSKgel® UP-SW2000 Size Exclusion Chromatography Column In-Line with LenS3TM MALS Detector
The Application Notebook
Biotherapeutics are generally larger molecules such as peptides, proteins and monoclonal antibodies with monomer molecular weight ranging from 3,000 to 150,000 Da. The drug must remain free from impurities such as fragment, dimer, and other higher order aggregates because they may cause severe immunogenic response. This becomes even more important particularly if the biotherapeutic protein is thermally susceptible. Historically, size exclusion is the preferred mode of chromatography for separation and characterization of such applications. Here we report the online detection of absolute molecular weight of two recombinant protein samples using a 2 μm size exclusion chromatography (SEC) column directly connected to the LenS3 Multi-Angle Light Scattering (MALS) detector.
Materials and Methods
Samples: BSA(Calibration standard)
Sample 1 – Recombinant proteins (~90 kDa) at 1.72 mg/mL in mobile phase
Sample 2 – Recombinant proteins (~90 kDa) at 3.64 mg/mL in mobile phase
The samples were stored at -20 °C and thawed to 8 °C just before analysis. The concentration was adjusted by diluting the sample in mobile phase pre-chilled at 8 °C.
Chromatographic Conditions
Instrument: ThermoFisher Ultimate® 3000 UHPLC and Chromeleon® software
Column: TSKgel UP-SW2000, 2 μm, 4.6 mm ID × 30 cm
Mobile Phase: BupH modified Dulbecco’s phosphate buffer prepared in light scattering grade water and filtered through a 0.1 μm PES membrane (The buffer was prepared from saline packs as per directions contained in Thermo Scientific– catalog # 28374, lot TL275790)
Flow Rate: 0.20 mL/min
Detectors: UltiMate 3000 VWD variable wavelength detectors @ 280 nm wavelength and Tosoh LenS3 MALS detector (positioned in series: Column → UV → MALS)
Column Oven Temp.: 25°C
Autosampler temp.: 8 °C
Injection Volume: Sample 1: 25 μL, Sample 2: 10 μL
Results and Discussion
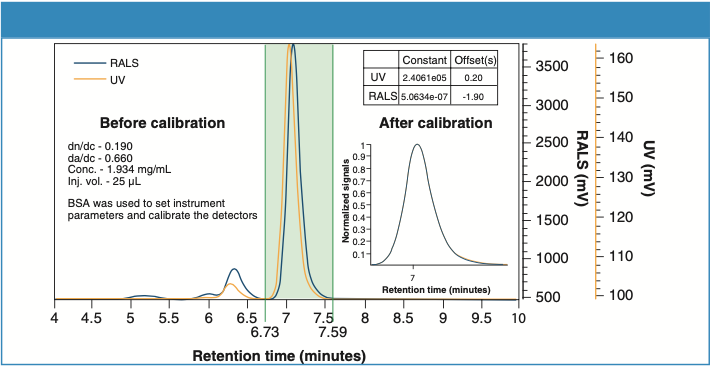
The multi-detector setup was calibrated using a freshly prepared bovine serum albumin (BSA) solution. Figure 1 shows the overlay trace of UV and MALS detectors. The one-step calibration procedure in the SECviewTM software adjusted the dead volume between the detectors and corrected for the band-broadening effect caused by the in-series detector configuration while determining the detectors’ calibration constants and offsets.
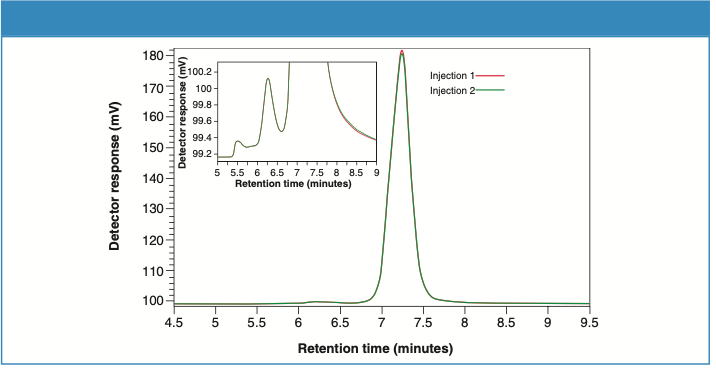
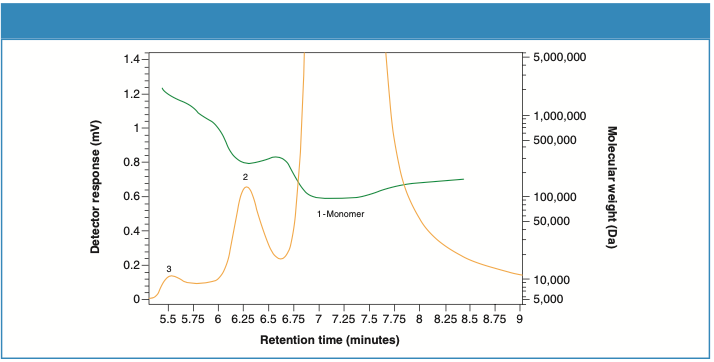
Figures 2 and 3 illustrate the UV detector overlays for two consecutive injections of Sample 1 and Sample 2, respectively. Zoomed-in figures (inset) show the excellent separation resolution between the monomer and the aggregates obtained from TSKgel UP-SW2000 column.
Figure 2: UV detector overlay for Sample 1 (two consecutive injections).
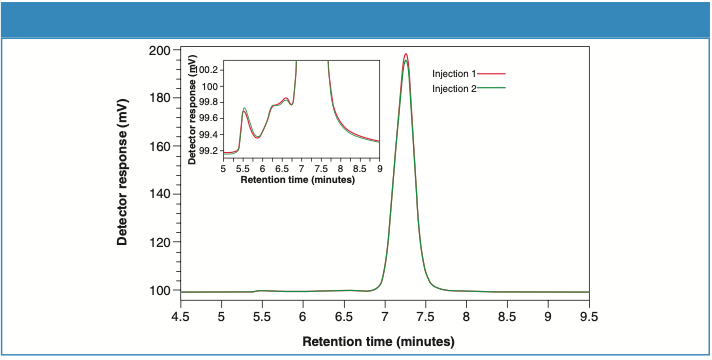
Figure 3: UV detector overlay for Sample 2 (two consecutive injections).
Figures 4 and 5 demonstrate the molecular weight (green) profiles for Sample 1 and Sample 2, respectively. The concentrations of the aggregate contents differ in the two samples.
Figure 4: Molecular weight profile for Sample 1.
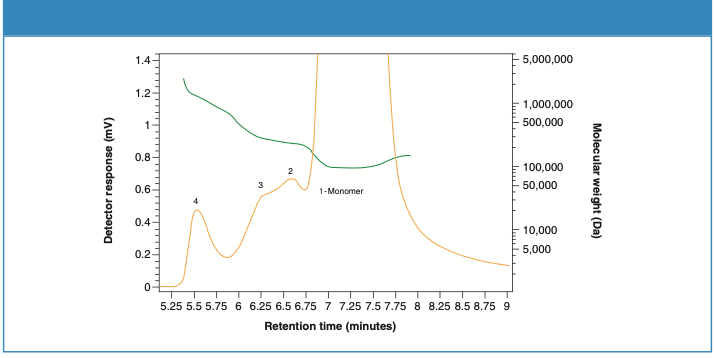
Figure 5: Molecular weight profile for Sample 2.
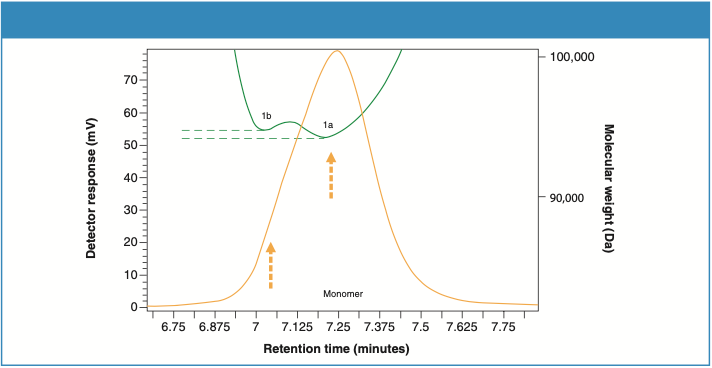
Looking closer at the UV trace, it appears that the monomer peaks in the samples illustrate a slight shoulder on the higher retention region, suggesting a bimodal shape. Further analysis using the molecular weight trace by the MALS detector reveals two separate populations of molecular weights. Figures 6 and 7 zoom in on the monomer peaks and demonstrate the two molecular weight plateaus, 1a and 1b, in both samples. Considering the sensitive nature of these recombinant proteins to the ambient conditions, the shoulder peak (1b) suggests the beginning of temperature-induced modifications, which varies in extent in both samples.
Figure 6: Molecular weight profile of the monomer peak for Sample 1.
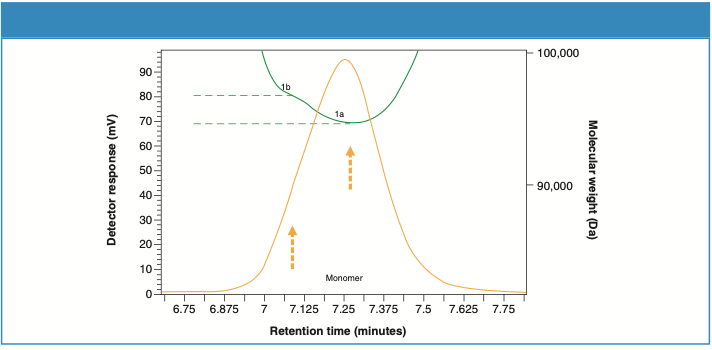
Figure 7: Molecular weight profile of the monomer peak for Sample 2.
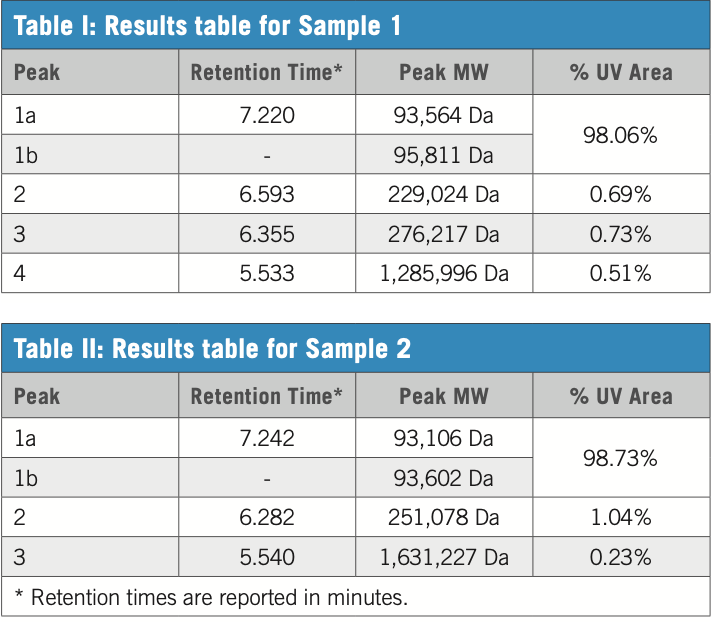
Tables I and II list the results of the chromatography analysis, including molecular weight and percent content, for the identified aggregate peaks.
Conclusions
This study shows that the molecular weight species including monomer and multiple aggregate levels present in the recombinant protein therapeutics could be determined and quantified using a SEC-MALS configuration. The TSKgel UP-SW2000 demonstrates excellent separation of the higher order aggregates from the monomer in both Sample 1 and Sample 2, as well as the slightly higher molecular weight temperature-induced variants that are almost co-eluted with the monomer. The column yields reproducible results, allowing accurate and precise chromatograms to be analyzed using the LenS3 MALS detector. The LenS3 accompanied by the SECview software also produces reproducible, accurate results in terms of MW determination for all peaks, in addition to area calculations, even at extremely low concentrations or in the presence of the aggregates.
TSKgel and Tosoh Bioscience are registered trademarks of Tosoh Corporation.
LenS is a trademark of Tosoh Bioscience LLC.
SECview is a registered trademark of Tosoh Bioscience LLC in the USA and EU and of Tosoh Corporation in Japan. UltiMate and Chromeleon are registered trademarks of the Dionex Corporation.

Tosoh Bioscience LLC
3604 Horizon Drive, Suite 100, King of Prussia, PA 19406
Tel. (484) 805-1219, fax (610) 272-3028
Website: www.tosohbioscience.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





