Automating Mycotoxin Analysis in Beverages
The analysis of hazardous mycotoxins is crucially important in food to ensure the health of humans and animals. Here, a simple and fast analysis including the sample preparation and purification of mycotoxins within food is presented. Ten different mycotoxins were investigated simultaneously in 14 min. The method was applied to the analysis of mycotoxins in apple juice, grape juice, and two different batches of the same branded beer.
Photo Credit: artjazz/Shutterstock.com

Carola Schultz, Shimadzu Europa, Duisburg, Germany
The analysis of hazardous mycotoxins is crucially important in food to ensure the health of humans and animals. Here, a simple and fast analysis including the sample preparation and purification of mycotoxins within food is presented. Ten different mycotoxins were investigated simultaneously in 14 min. The method was applied to the analysis of mycotoxins in apple juice, grape juice, and two different batches of the same branded beer.
Mycotoxins are hazardous to humans. They are secondary metabolites produced by fungi (1). Mycotoxins can be divided into several groups. This study focuses on aflatoxins, ochratoxin, and patulin.
Aflatoxins are produced by fungal molds of the Aspergillus species, which prefer a warm and damp climate. They are often associated with commodities produced in the subtropics and tropics, like peanuts, cotton, spices, or pistachios. Furthermore, they can be produced by fungal infestation during or after harvest of grain and can end up in processed products such as beer, brewed from malt.
Toxic and Carcinogenic
Aflatoxins are not only toxic but also known to be carcinogenic. Aflatoxin B1 is the most common type of Aflatoxin present in food products, and it is also one of the most genotoxic and carcinogenic species (2). Ochratoxin is produced by Penicillium and Aspergillus species. It is a contaminant in beverages such as beer and wine. Patulin, produced by P. expansum, Aspergillus, Penicillium, and Paecilomyces fungal species, is often found in mouldy fruits and vegetables. Zearalenone is produced by Fusarium species and may be present in crops; AFM1 is often found in milk products.
Investigation of 10 Mycotoxins
To ensure food safety, manufacturers of food and beverages must strictly manage risks from such contaminants, so sensitive methods to assay mycotoxins in complex matrices are essential. Mycotoxins are not destroyed by temperature treatment, and are barely influenced by cooking, freezing, or digestion. These properties make the investigation in food crucially important as consumers need to be protected from these toxic substances.
AFB1, AFB2, AFG1, AFG2, AFM1, OTA, ZON, DON, NIV, and PAT were investigated. For this approach, the five analytes most commonly tested in malt products were extracted and analyzed in spiked and nonâspiked beer samples from different batches, as well as PAT in apple juice and grape juice. EU limit values are: Aflatoxins (B1, B2, G1, and G2: total: 4–15 µg/kg; AFB1: 2–12 µg/kg); AFM1: 0.05 µg/kg; OTA: 2–10 µg/kg; PAT:
25–50 µg/kg; DON: 500–1750 µg/kg; NIV: not specified; ZON: 20–400 µg/kg. Detection of the mycotoxins was conducted using a combination of fluorescence and photodiode array (PDA) detection.
Materials and Methods
Analytical Conditions: HPLC system: LC-2040C 3D (Shimadzu Corporation); fluorescence detector: RF-20AXS: Ex (nm): 365 nm and 320 nm; Em (nm): 450 nm and 465 nm; PDA: D2 at 190–500 nm, reference at 350 nm; column: 3.0 mm × 75 mm, 2-μm Shim-pack GIST C18 (Shimadzu); mobile phase: A: 20 mmol/L sodium phosphate buffer pH 2.5 (10 mmol NaH22PO4 and 10 mmol H3PO4); B: acetonitrile; C: methanol; flow rate: 1.0 mL/min; injection volume: 10 µL.
Sample Preparation: Two batches of beer, apple juice, and grape juice were the object of this investigation. The samples were prepared as non-spiked or spiked with mycotoxins. A 4-g measure of the beverages was mixed with 21 mL of acetonitrile. The mixtures were cleaned with a multifunctional column (MultiSep 228 AflaPat, cartridge type [syringe format cleanup column] purchased from Romer Labs). Four mL of the elution were collected and evaporated to dryness with nitrogen gas. The samples were then reâdissolved in a 400 µL water–acetonitrile (95/5, v/v) solution and used directly for analysis. A standard mixture containing 10 different mycotoxins (AFB1, AFB2, AFG1, AFG2, AFM1, OTA, ZON, DON, NIV, and PAT) was prepared.
Results
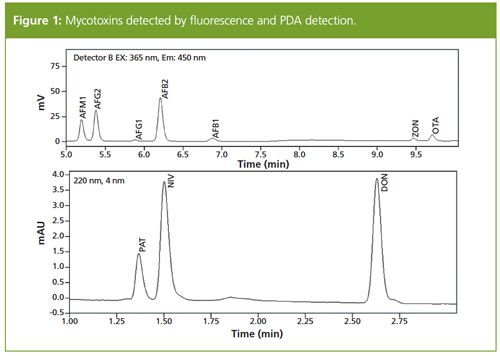
Mycotoxins in Apple Juice, Grape Juice, and Two Different Batches of Beer: The standard mixture including all 10 mycotoxins was analyzed and the chromatogram is shown in Figure 1. All 10 mycotoxins were successfully separated from each other and detected by fluorescence detection and PDA.
This method was applied to different non-spiked and spiked beverage samples (Figure 2). In all spiked beverage samples, the mycotoxins were successfully identified despite the complexity of the matrices.
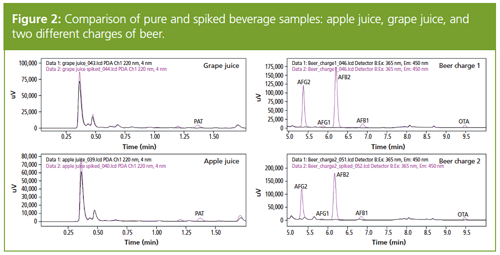
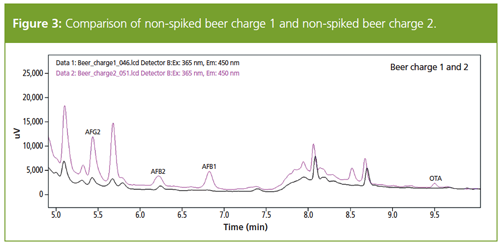
Figure 3 compares the different beer batches. The beer sample of batch 2 showed small peaks for AFG2, AFB2, AFB1, and OTA. Even though the concentration of the mycotoxins is far below the EU maximum residue limits, this is a clear indication of the presence of mycotoxins in the beer of batch 2.
EU Maximum Residue Limits and LODs and LOQs: The EU maximum residue limits for mycotoxins as specified by EU standards are the strictest in the world (3–5). To check whether the food samples investigated contain mycotoxins within the EU maximum residue limits, standards with the mycotoxins were prepared using the same concentration as that of the EU control criteria. A simple and fast oneâpoint calibration was enough to
assess compliance with the criteria. A batch was created with the calibration standard and the food samples, and the results show if the peaks of the mycotoxins in the food samples were below or above the criteria.
In this study, all measured samples (apple juice, grape juice, and two batches of beer) either contained no mycotoxins or were in a range far below the EU criteria. The same, spiked samples showed mycotoxin concentrations above the EU criteria.
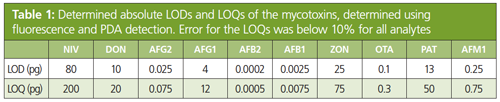
For all mycotoxin standards, the limit of detection (LODs) and limit of quantitation (LOQs) were determined as shown in Table 1. The LODs and LOQs were low and at least half of the EU maximum residue limits.
Conclusion
This study shows the applicability of an automated screening system from pretreatment to analysis and final reporting of analytical results for the simultaneous analysis of 10 commonly tested mycotoxins: AFB1, AFB2, AFG1, AFG2, AFM1, OTA, ZON, DON, NIV, and PAT. Using a combination of fluorescence and photodiode array (PDA) detection, detection limits below the EU maximum residue limits were provided for all compounds of interest (3–5). This approach offers a whole solution from sample pretreatment to results. Those unfamiliar with LC systems or with little chromatography experience can benefit from this approach.
The five analytes most commonly tested in malt products were extracted and analyzed in spiked and non-spiked beer samples from different batches, as well as PAT in apple juice and grape juice. The method for analysis of mycotoxins was used successfully for the analysis of beer, apple juice, and grape juice. Sample preparation techniques as well as measurement were easy and fast. The results showed that the grape juice, apple juice, and beer of batch 1 contain mycotoxins below the detection limits. Interestingly, the beer of batch 2 contained the mycotoxins AFG2, AFB2, AFB1, and OTA.
The level of these mycotoxins was below the European regulation. The results show that different batches of the same beer show differences in mycotoxin content. Within this study, a fast, safe, and easy method was shown for the analysis of mycotoxins in beverages. A further advantage, especially for food quality control analysis, is the simultaneous separation of all 10 mycotoxins within only 14 min.
References
- J.L. Richard, J. Food Microbiol.119(1–2), 3–10 (2007).
- European Food Safety Authority: http://www.efsa.europa.eu/de/topics/topic/aflatoxins
- EU: Commission Regulation (EC) No 1881/2006 of 19 December 2006 (consolidated version 2010-07-01). Setting maximum levels for certain contaminants in foodstuffs.
- EU: Commission Regulation (EC) No 165/2010 of 26 December 2010 amending Regulation (EC) No 1881/2006. Setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins.
- EU: Commission Regulation (EC) No 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006. Setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A.
Carola Schultz graduated with a M.Sc. in chemistry at the University of Münster (Germany) in 2013. Her PhD subject work was completed (title award to follow) at MEET Battery Research Center of the Institute of Physical Chemistry (University of Münster) and focused on analytical chemistry and mainly high performance liquid chromatography (HPLC). Since 2016, she has worked as a product specialist for consumables in the Center of Innovation and Product Support at Shimadzu Europa in Duisburg, Germany.
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.










