Dietary Supplements Method Modernization—A Review of Current Efforts to Modernize Compendial Methods and a Summary of Allowable Adjustments
The Column
The implementation of the FSMA (Food Safety Modernization Act) and the continued globalization of the food supply have increased the need for improved analytical methods for quality and safety testing. Food safety and quality requirements are increasingly stringent, as are consumer expectations. In addition to new methods, the industry continues to look for improvements in laboratory productivity and new ways to reduce costs. These trends extend to dietary supplements and natural products. This article discusses the current state of efforts to modernize US Pharmacopeia (USP) compendial methods to take advantage of new chromatography technology, including the use of “allowable adjustments”.
Photo Credit: tadamichi/Shutterstock.com

Philip J. Koerner, Scott Krepich, and David Kennedy, Phenomenex, Torrance, California, USA
The implementation of the FSMA (Food Safety Modernization Act) and the continued globalization of the food supply have increased the need for improved analytical methods for quality and safety testing. Food safety and quality requirements are increasingly stringent, as are consumer expectations. In addition to new methods, the industry continues to look for improvements in laboratory productivity and new ways to reduce costs. These trends extend to dietary supplements and natural products. This article discusses the current state of efforts to modernize US Pharmacopeia (USP) compendial methods to take advantage of new chromatography technology, including the use of “allowable adjustments”.
The implementation of the FSMA (Food Safety Modernization Act) and the continued globalization of the food supply have brought new focus to the need for improved analytical methods for testing food (1). Better methods are needed to meet increasingly stringent safety and quality requirements and heightened consumer expectations. At the same time, the industry is looking for innovations to improve laboratory productivity and reduce testing costs as manufacturers and suppliers strive to maintain profitability while meeting these new safety and quality requirements. Perhaps nowhere is the need for improvement more evident than in the area of dietary supplements and natural products.
The Need for Modern HPLC Methods
Chromatography-primarily high performance liquid chromatography (HPLC) analysis-has long been the principal tool to qualitatively and quantitatively characterize dietary supplements. HPLC can identify and quantify the essential ingredients in complex botanical matrices and, as such, has served the dietary supplements industry well. This is witnessed by the large number of “official” or compendial methods that are in everyday use. Unfortunately, many of these widely used methods for dietary supplements were developed many years ago-sometimes decades ago-based on HPLC technology that is now quite dated. In the last decade, a wide variety of improvements have been made in HPLC particle morphology and chemistry that have greatly enhanced the ability to resolve complex mixtures. This has provided a superior basis to accurately measure active ingredients, while also detecting impurities and potential adulteration. Add to this the great improvements in analytical instrumentation (such as ultrahigh-pressure LC [UHPLC]), which have dramatically increased analytical resolution and speed, and it is apparent that compendial chromatographic methods for dietary supplements would greatly benefit from the application of current technology. Hence, the need for modernization. This need has been recognized by the US Pharmacopeia (USP), who in 2016 set a 5-year goal to modernize the several hundred USP chromatographic methods for dietary supplements.
The SoCal Dietary Supplements Chromatography Forum
Changing, modifying, or “modernizing” a compendial method is a complex technical challenge. Therefore, modernization is not a task to be undertaken lightly. In recognition of both the need and the challenge of modernization, USP, Phenomenex, and Alkemist Laboratories hosted the SoCal Dietary Supplements Chromatography Forum in July 2016. This assembly was designed to collaboratively discuss the need and approach to modernizing USP dietary supplement monographs, particularly those based upon chromatography.
The event drew over 40 industry experts and featured lecture topics on such topics as:
- Advancements in chromatography for dietary supplements
- Allowable adjustments to USP methods
- A modern approach to compendial testing for dietary products.
USP Scientific Liaison, Anton Bzhelyansky, presented a talk on updating spectrophotometric methods to chromatographic methods in compendial monographs. Following the presentations, forum participants gathered in small groups to discuss three questions of critical importance to the dietary supplements industry:
- What methods are in the greatest need of modernization, and why?
- What challenges are there to modernize these methods under the USP allowable criteria?
- How would the method modernization actually be accomplished or implemented?
The consensus of the group was that the methods in greatest need of modernization are:
- Fatty acid profile
- Total, soluble, and insoluble dietary fibre
- Macronutrients
- Carotenoids
- Boswellia
- Chondroitin
- Multivitamins
- The conversion of titration and spectrographic methods to HPLC.
Sample complexity, standards availability, and validation constraints were also listed as challenges of modernization, among many others. USP representatives gave critical feedback to attendees on these questions and the enthusiastic participation of the attendees confirmed the interest of the dietary supplement community for modernizing dietary supplement testing methods.
A Collaborative Approach to Modernization
As a result, a project was commissioned by Phenomenex, Alkemist Labs, and USP to create a pilot method modernization programme using the USP Boswellia serrata Monograph as the model. The goal of the project is to update the analytical chromatography of the monograph to current technology levels, thereby demonstrating the effectiveness of a collaborative method modernization project between USP and industry. The modernization approach taken was to employ HPLC and UHPLC coupled to ultraviolet (UV) for the analysis of Boswellic Acids in plant-derived resins, including these primary species: Boswellia serrata Roxb Boswellia sacra Flueck, Boswellia papyrifera, Boswellia frereana Birdw, and secondary species Boswellia neglecta S.Moore and Boswellia rivae. Testing criteria included both identification and quantitation of total and individual acids using standards and authentic plant materials. The objective of the pilot programme is to demonstrate a collaborative process to modernize current high-use, compendial dietary supplement methods. If successful, this collaborative process could be used as a model for the modernization of other compendial methods.
The Boswellia Method Modernization Project
The project was initiated with a review of the current USP compendial method along with many other publications for Boswellia serrata. Several column chemistries and conditions were screened with the goal of developing an optimized HPLC or UHPLC method for standards and extracts.
The current USP method (Figure 1) utilizes a fully porous 5-µm C18 column in traditional HPLC dimensions (250 × 4.6 mm). The method quantifies total boswellic acids as four individual standard analytes:
- 11-keto-β-boswellic acid (KBA)
- 3-acetyl-11-keto-β-boswellic acid (AKBA)
- β-boswellic acid (βBA)
- 3-acetyl-β-boswellic acid (AβBA).
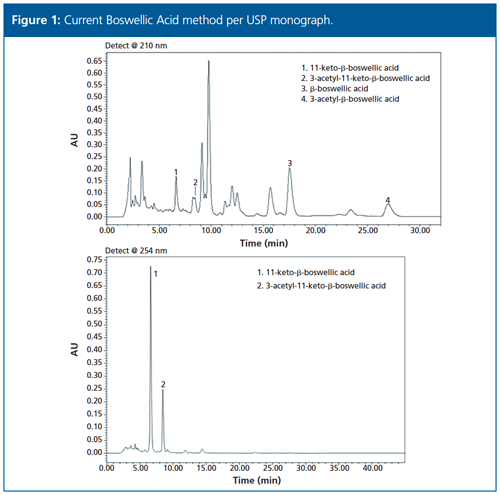
The method features a 45-min runtime and dual wavelength measurements at 210 nm and 254 nm. The 254 nm wavelength is used to quantify KBA and AKBA, which coelute with matrix interferences at the 210 nm wavelength.
The primary goals of the modernized UHPLC method are to reduce the chromatographic runtime and to include two additional boswellic acid analytes: α-Boswellic acid (αBA) and 3-Acetyl-α-boswellic acid (AαBA).
Several core–shell and fully porous column chemistries were screened (Figure 2) in a 100 × 3.0 mm column dimension with an eye towards flexibility with both UV and mass spectrometry detectors, along with a diverse range of front-end HPLC and UHPLC instruments.
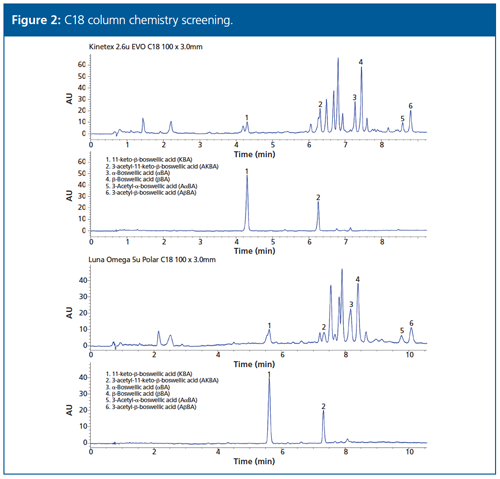
These changes successfully reduced the runtime to under 10 min with similar selectivity for the original four boswellic acids, while also providing complete separation of the two additional boswellic acid standards-αBA and AαBA. Boswellic acid, KBA, and AKBA still coelute with some matrix interferences and require a second quantitation at the 254 nm wavelength.
Experimental: Initial screens were performed at Alkemist Labs on a Waters Acquity UPLC H-Class instrument with Boswellia Serrata Extract and 3-Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) USP reference standards provided by the USP, and the remaining identifications from individual certified reference standards from Extrasynthase (11-keto-β-boswellic acid (KBA), β-boswellic acid (βBA), 3-acetyl-β-boswellic acid (AβBA), α-Boswellic acid (αBA), and 3-Acetyl-α-boswellic acid (AαBA). Additional screens were performed at Phenomenex on an Agilent 1100 HPLC instrument.
Complementary phenyl column chemistries were also explored in core–shell platforms (Figure 3). The pentafluorophenyl column did not look promising. The biphenyl and phenylâhexyl columns demonstrated good resolutions and peak shapes for most components, although with significantly different profiles and elution orders compared to alkyl phase and to each other. This suggests that there is opportunity for improvement with further exploration and optimization.
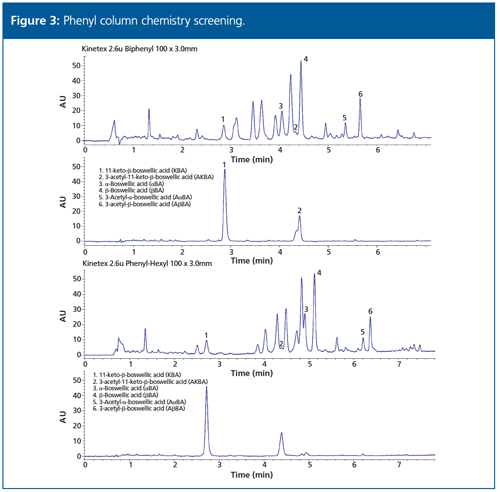
Follow Up: Further optimization and specificity work is in progress for the complete modernization of the monograph. When the project is completed, the full results will be shared with the dietary supplements community in an appropriate scientific forum, and additional collaborative method modernization projects will be initiated. Dietary supplement manufacturers and contract laboratories will be given the opportunity to participate in future method modernization efforts.
Meanwhile, prior to that time when compendial methods are modernized and released, there is an excellent alternative procedure available to improve USP methods. This is known as the Allowable Adjustment Process and can permit the use of available particle and morphologies without going through a revalidation process.
Allowable Adjustments to Pharmacopoeial HPLC Methods
Effective 1 August 2014, the United States Pharmacopeia (USP) published the latest revision to General Chapter <621> on Chromatography that provides clear guidance regarding what “allowable adjustments” can be made to USP methods without having to revalidate these methods. Understanding this latest revision can help improve productivity and cut costs in any laboratory environment.
Generally, USP monographs were developed for generic drugs and dietary supplements, and many of these methods were developed on older materials and are time-consuming to run. With the availability of much higher efficiency HPLC and UHPLC columns, many of these methods could be improved to increase productivity in the laboratory and reduce the lengthy runtimes often associated with these methods, while maintaining or improving chromatographic resolution and meeting the system suitability requirements defined in the monograph.
Allowable Adjustments and System Suitability
Before we review the allowable adjustments, let’s summarize some of the key points of USP <621>:
1. Allows for adjustments to methods (and defines the maximum allowable adjustments):
- “in order to comply with system suitability”
- when “it may be desirable to use a column with different dimensions”
- “only when suitable standards (including reference standards) are available for all compounds used in the suitability test and the adjustments yield a chromatogram that meets all the system suitability requirements specified in the official procedure”
- “are not to be made in order to compensate for column failure or system malfunction”
2. Adjustments may require additional validation
3. Multiple adjustments should be considered carefully
4. Addresses the continued trend towards <3 μm particles, superficially porous (core–shell) particles, and fast LC and UHPLC.
The maximum allowable adjustments are outlined in Table 1 for isocratic methods, and Table 2 for gradient methods (more limited). Adjustments may require verification, and multiple adjustments should be considered carefully because they can have a cumulative effect on system performance. Changes in the chemical characteristics of the stationary phase, such as a change from L1 (C18) to L7 (C8), are considered a modification and require revalidation.
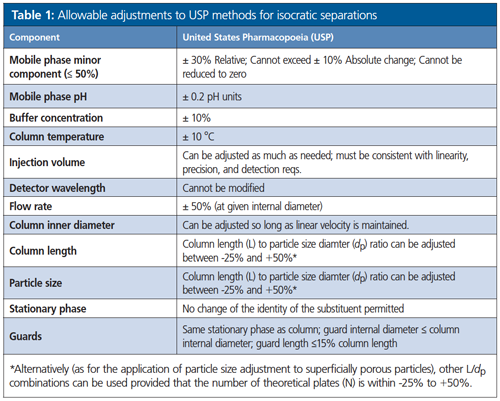
Adjustments to Column Length and Particle Size
For isocratic separations only (not allowed for gradient separations), the ratio of column length (L) to the particle size (dp) must remain constant or within a range between -25% to +50% of the prescribed L/dp ratio. Alternatively, other combinations of L and dp (for example, for the application of particle size adjustment to superficially porous particles [aka core–shell particles]) can be used provided that the number of theoretical plates (N) is within - 25% to +50%, relative to the prescribed column.
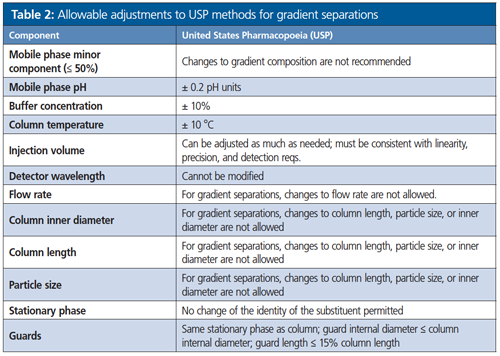
Guard Columns
The use of guard columns will protect valuable analytical columns by removing particulates and strongly retained sample components that may accumulate. This can increase column lifetimes and help maintain performance (efficiency, resolution, and peak shape). This latest revision clearly addresses the use of guard columns. Specifically, a guard column may be used with the following requirements, unless otherwise indicated in the individual monograph:
a) Guard column length must be ≤15% the length of analytical column
b) Inner diameter must be the same or smaller than the analytical column
c) The packing material should be the same as the analytical column (for example, silica) and contain the same bonded phase (C18).
When using a guard column, all system suitability requirements specified in the official procedure must be met.
In conclusion, USP General Chapter <621> defines the maximum “allowable adjustments” to compendial methods. These adjustments permit flexibility for users of compendial methods to greatly increase productivity in the laboratory by ultimately reducing runtimes, while also reducing solvent usage and cutting costs. System suitability must be met or revalidation will be required. Finally, USP <621> also allows for guard columns if stated requirements for their use are met.
Dietary supplement label claim verification is a continual concern for the industry and its consumers. Therefore, manufacturers and contract laboratories are under pressure to use the most accurate, reproducible, and efficient methods. Older compendial methods are still widely used, but there is a great need for method modernization for productivity, costâeffectiveness, quality, and safety purposes.
Acknowledgements
Special acknowledgements and thanks to USP, Alkemist labs, and Extrasynthese for their support and contributions. The combination of technical expertise, standards and extracts, chromatography consumables, and laboratory resources was crucial in demonstrating the potential of a joint collaboration to support the modernization of compendial methods.
Reference
- http://www.fda.gov/Food/GuidanceRegulation/FSMA/ucm247546.htm
Phil Koerner is a senior technical manager at Phenomenex and has more than 27 years of industry experience in LC, GC, and sample preparation techniques.
David C. Kennedy is a business development manager for Phenomenex.
Scott Krepich is a field application scientist for Phenomenex.
E-mail:AllenM@phenomenex.com
Website:www.phenomenex.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













